
What is the compound that contains the ring structure of benzene called?
Answer
492.3k+ views
Hint: The most complex aryl hydrocarbon is benzene. Each carbon atom in the benzene ring has two carbon-carbon sigma bonds, one carbon-hydrogen sigma bond, and one delocalized pi electron double bond with a neighbouring carbon.
Complete answer:
A compound that contains a benzene ring is known as an aromatic compound.
Compounds that contain a benzene ring were originally called aromatic compounds because they had a characteristic aroma or smell.
Aromatic compounds are made up of conjugated planar ring systems with delocalized pi-electron clouds in place of individual alternating double and single bonds.
Aromatic hydrocarbons are ring-shaped hydrocarbons with sigma bonds and delocalized pi electrons between carbon atoms. Consider the chemical benzene. They are called aromatic because of their pleasant smell.
Examples of aromatic compounds are:
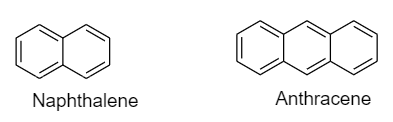
Benzenoids (chemicals with a benzene ring) and non-benzenoids (compounds without a benzene ring), such as furan, are the two types of aromatic compounds. If the Huckel rule is followed, any hydrocarbon can be categorised as an aromatic compound.
In water, aromatic compounds are typically nonpolar and non-miscible. These chemicals are typically nonreactive and serve as solvents for a variety of nonpolar molecules. Because their carbon to hydrogen ratio is high, they produce a sooty yellow flame.
Note:
Cyclic compounds can be aromatic or non-aromatic; for example, benzene is an aromatic cyclic compound, whereas cyclohexane is not. Aliphatic compounds are organic compounds that are not aromatic, but only aromatic rings are particularly stable.
Complete answer:
A compound that contains a benzene ring is known as an aromatic compound.
Compounds that contain a benzene ring were originally called aromatic compounds because they had a characteristic aroma or smell.
Aromatic compounds are made up of conjugated planar ring systems with delocalized pi-electron clouds in place of individual alternating double and single bonds.
Aromatic hydrocarbons are ring-shaped hydrocarbons with sigma bonds and delocalized pi electrons between carbon atoms. Consider the chemical benzene. They are called aromatic because of their pleasant smell.
Examples of aromatic compounds are:

Benzenoids (chemicals with a benzene ring) and non-benzenoids (compounds without a benzene ring), such as furan, are the two types of aromatic compounds. If the Huckel rule is followed, any hydrocarbon can be categorised as an aromatic compound.
In water, aromatic compounds are typically nonpolar and non-miscible. These chemicals are typically nonreactive and serve as solvents for a variety of nonpolar molecules. Because their carbon to hydrogen ratio is high, they produce a sooty yellow flame.
Note:
Cyclic compounds can be aromatic or non-aromatic; for example, benzene is an aromatic cyclic compound, whereas cyclohexane is not. Aliphatic compounds are organic compounds that are not aromatic, but only aromatic rings are particularly stable.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




