
Compound A (molecular formula \[{{C}_{5}}{{H}_{10}}\]) gives only one monochlorinated product. Write the structure of compound A.
1-pentene
2-pentene
1-methyl cyclobutane
cyclopentane
Answer
590.4k+ views
Hint: If any hydrocarbon contains primary and secondary hydrogen atoms then they will undergo chlorination and give at least two monochlorinated derivatives.
If the product has only secondary hydrogen atoms (means only one type of hydrogen atoms) then monochlorination is possible.
Complete step by step answer:
The given compound has a molecular formula of\[{{C}_{5}}{{H}_{10}}\].
So, the degree of unsaturation of organic compounds can be calculated using \[{{C}_{n}}{{H}_{2n+2}}\] for alkanes, \[{{C}_{n}}{{H}_{2n}}\] for alkenes or cycloalkanes, \[{{C}_{n}}{{H}_{2n-2}}\] for alkynes).
The molecular formula of the given hydrocarbon matches with \[{{C}_{n}}{{H}_{2n}}\] for alkenes or cycloalkanes.
The degree of unsaturation of the given compound is 1. It should have either one double bond or should be a cyclic compound.
To do chlorination or halogenation, the hydrocarbon should have either one double bond or it should be a cyclic compound.
If the hydrocarbon has one double bond, then it will give more than one monochlorinated product. So, the hydrocarbon should be a cyclic compound. Because in cyclic hydrocarbon only one type of hydrocarbons is present (mostly secondary hydrogen atoms are present).
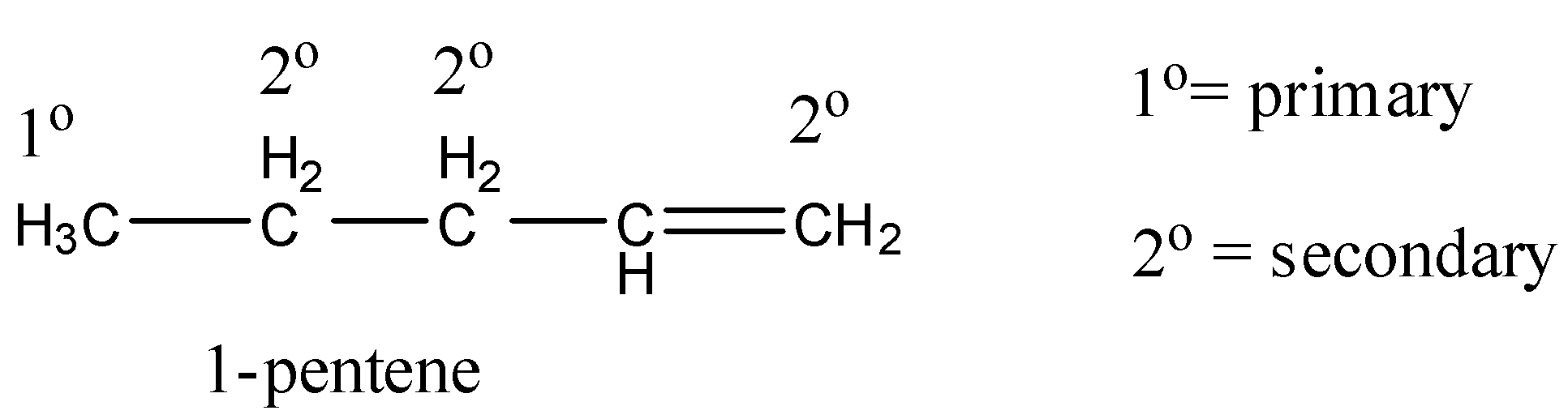
Coming to the given options, option A, 1-pentene (the structure is as follows).
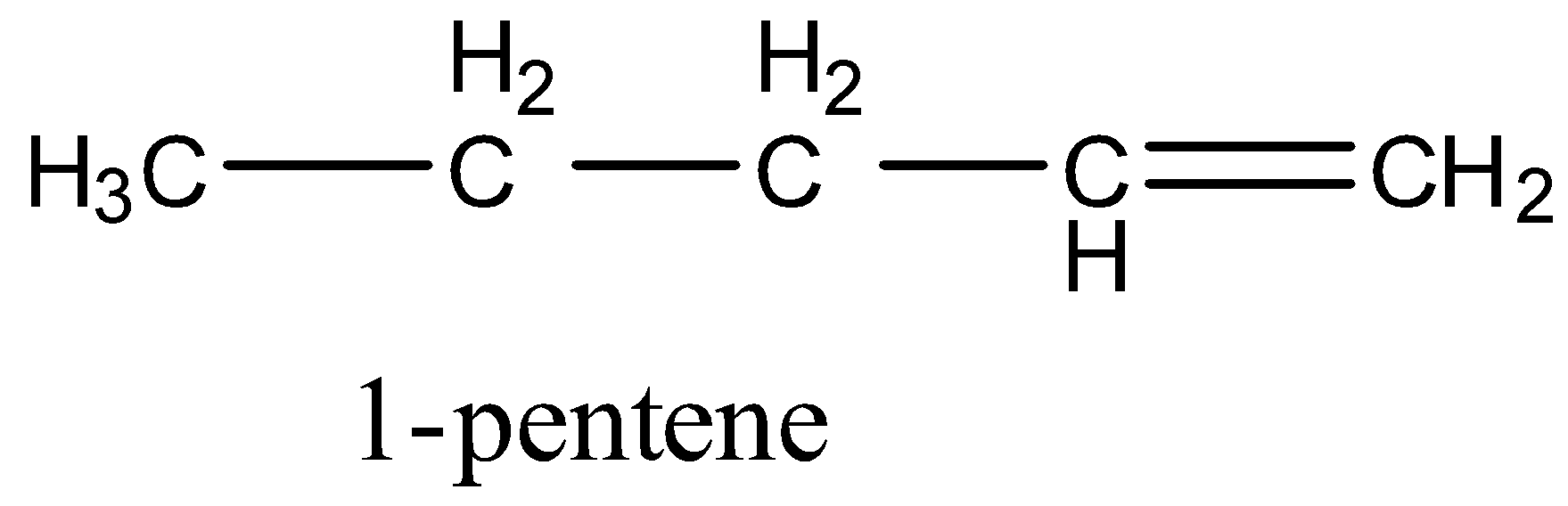
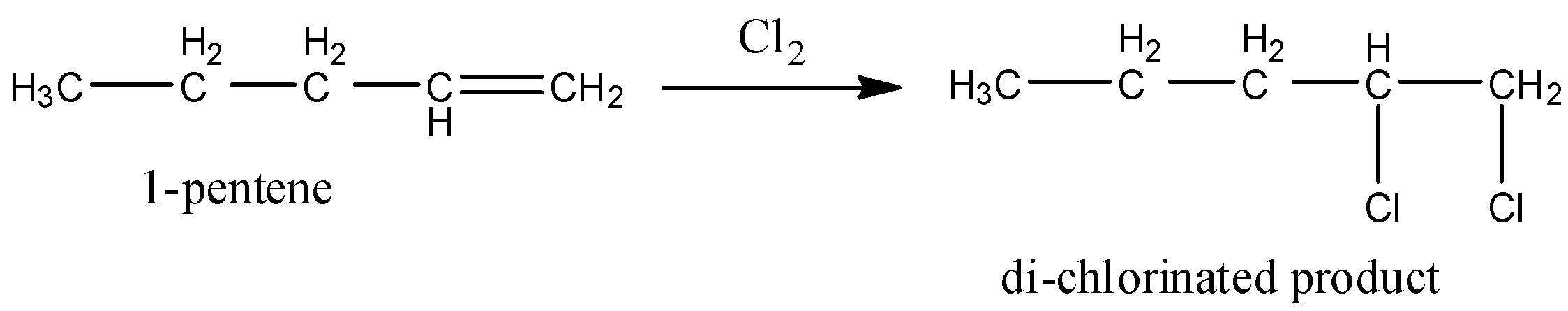
In 1-pentene, primary, secondary hydrogens and one double bond are present.
So, 1-pentene undergoes chlorination and gives a di-chlorinated product. Therefore option A is wrong.
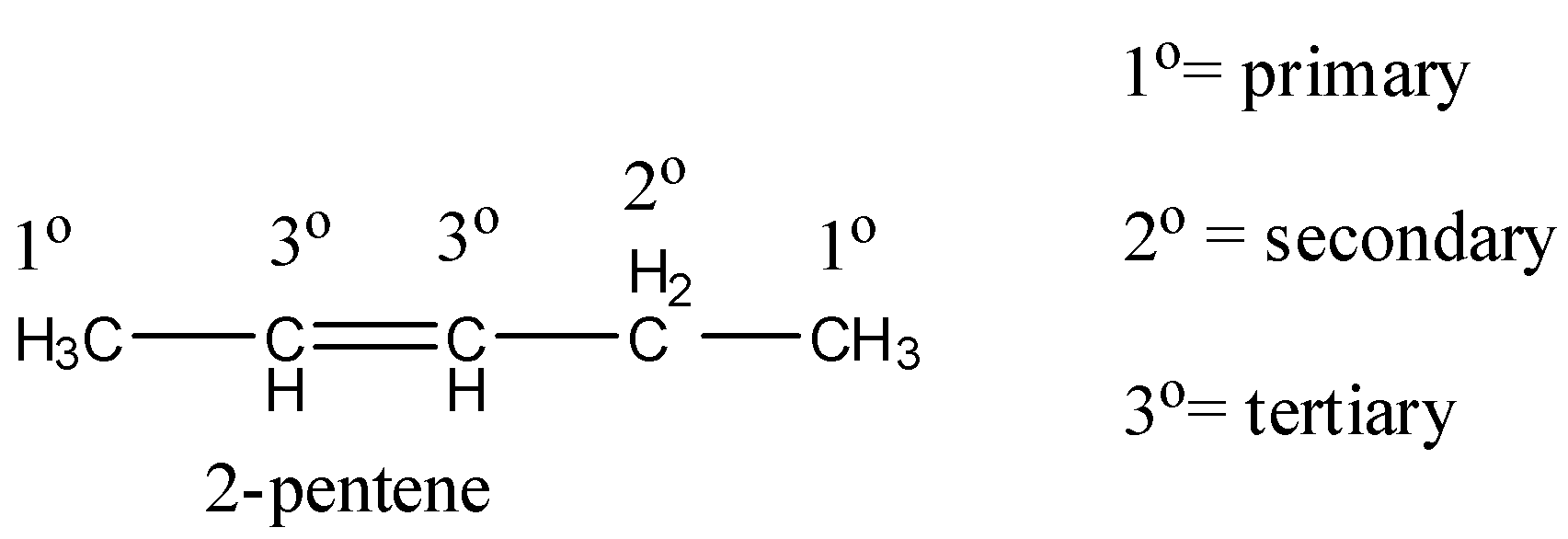
Coming to option B, 2-pentene (the structure is as follows).
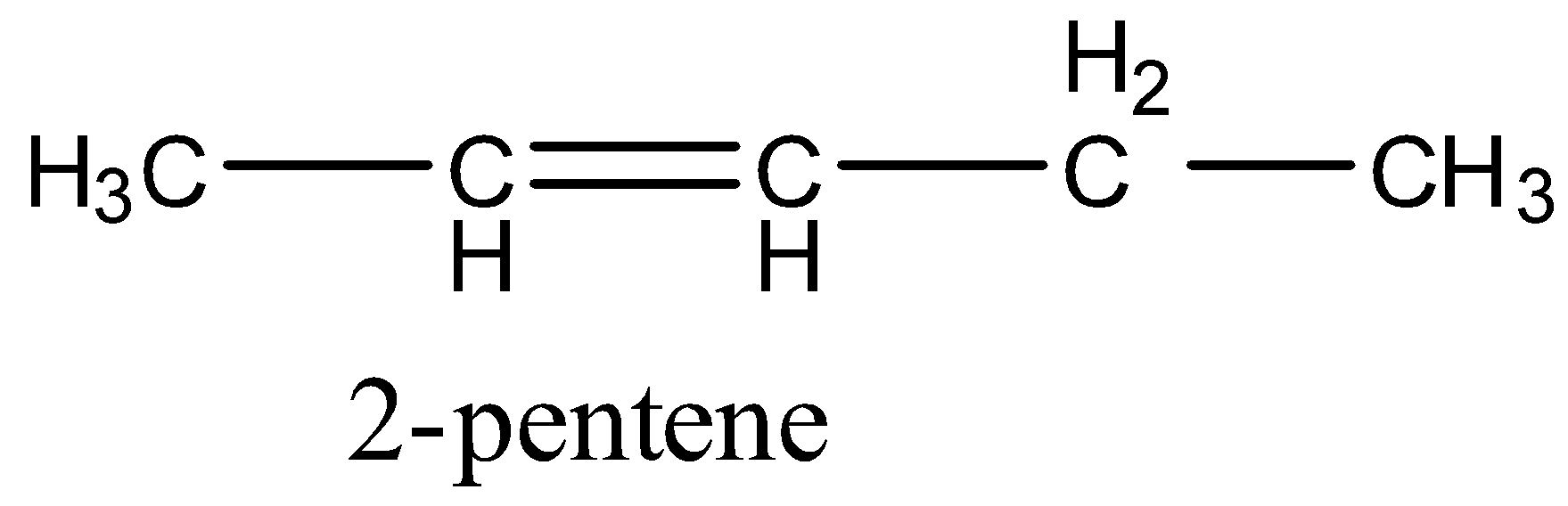
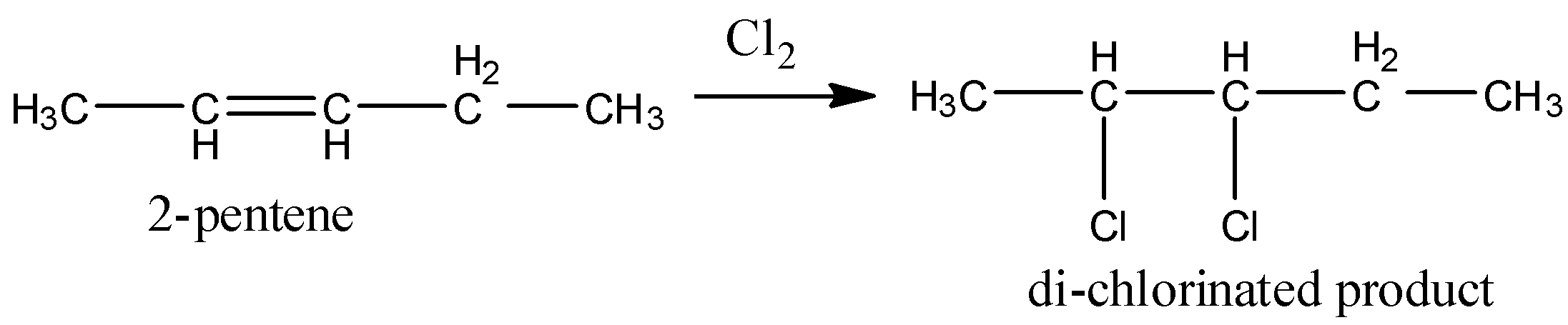
In 2-pentene, primary, secondary hydrogens and one double bond are present.
So, 2-pentene undergoes chlorination and gives a di-chlorinated product. Therefore option B is wrong.
Coming to option C, 1-methyl cyclobutane (the structure is as follows).
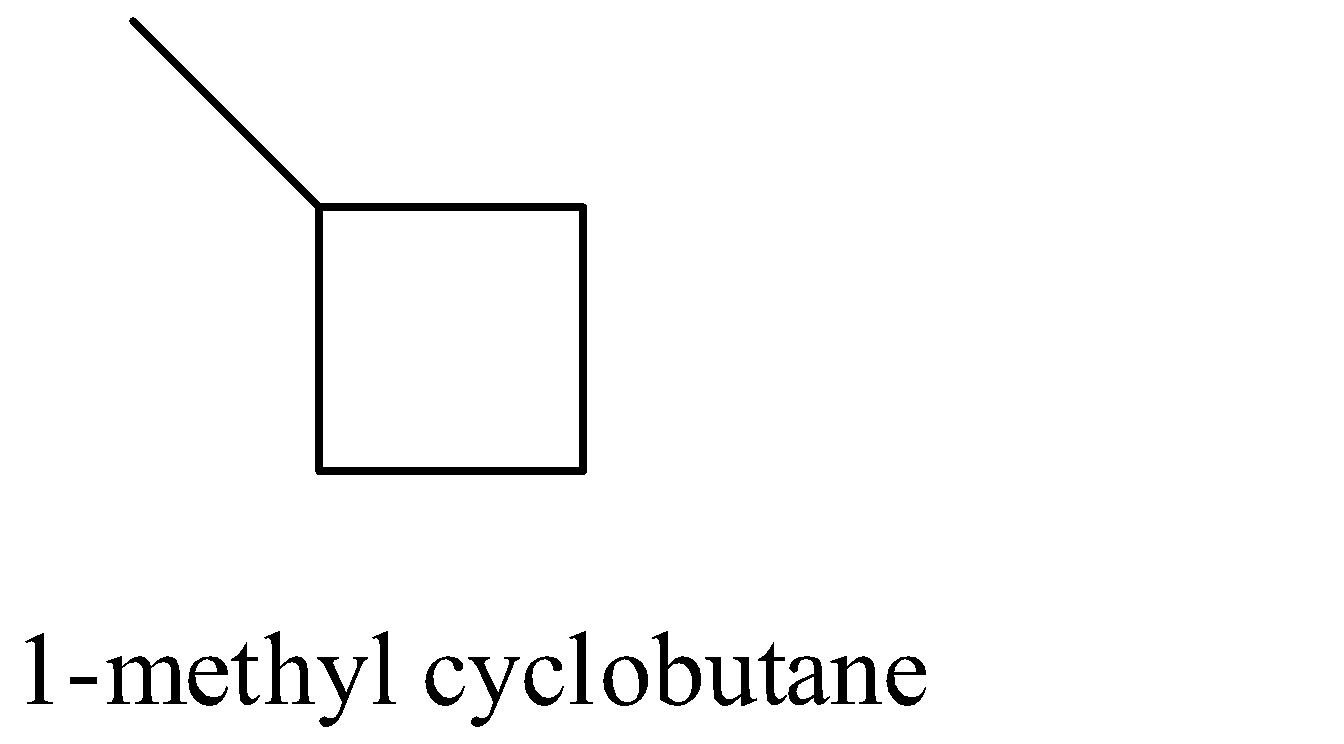
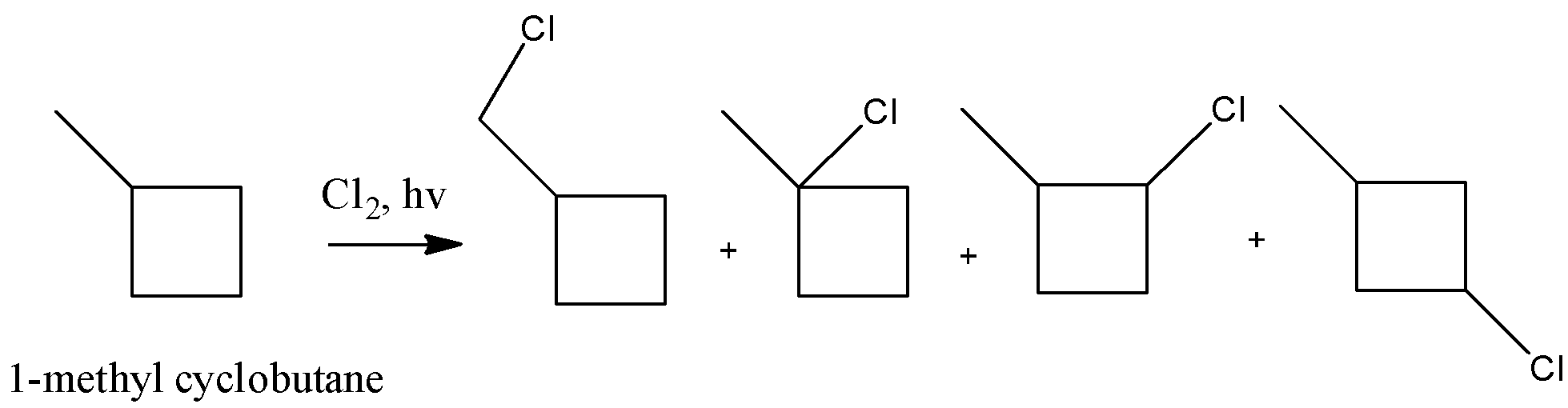
In 1-methyl cyclobutane, primary, secondary and tertiary hydrogens are present.
So, 1-methyl cyclobutane undergoes chlorination and gives more than one monochlorinated product. Therefore option C is wrong.
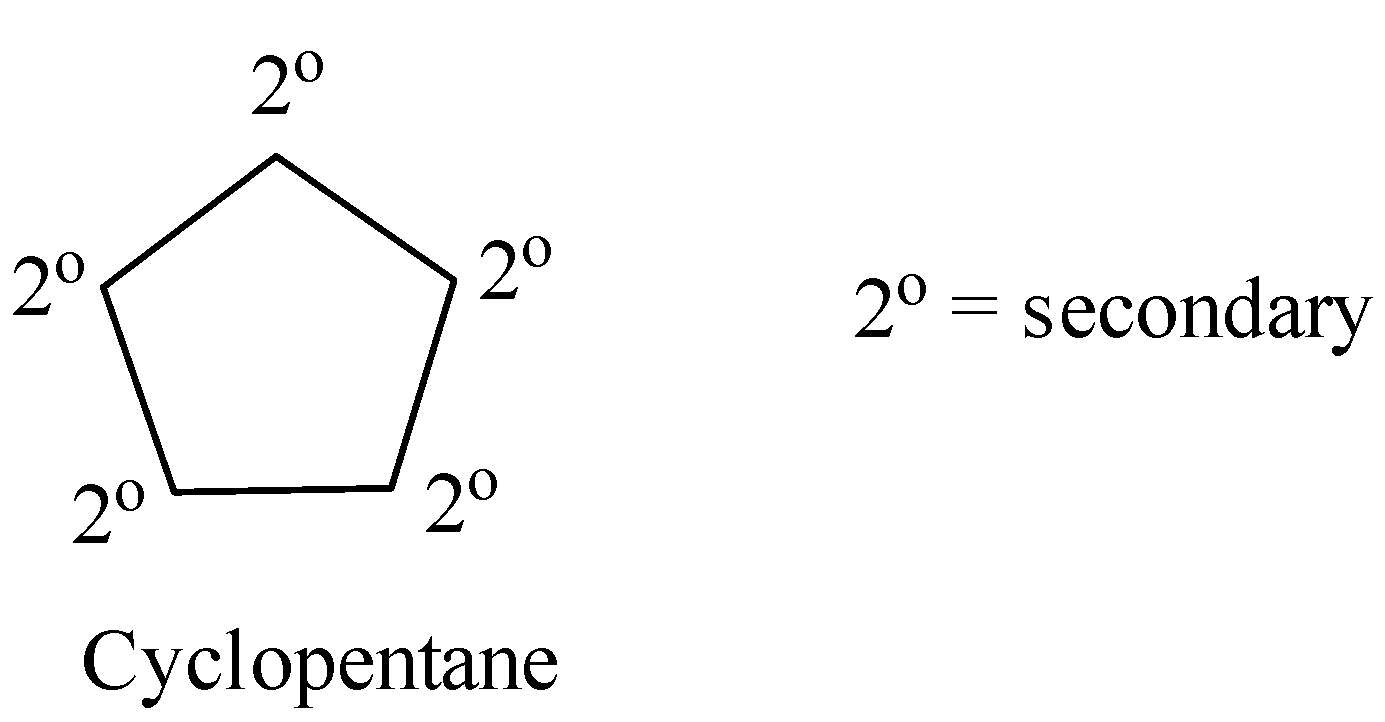
Coming to option D, cyclopentane.
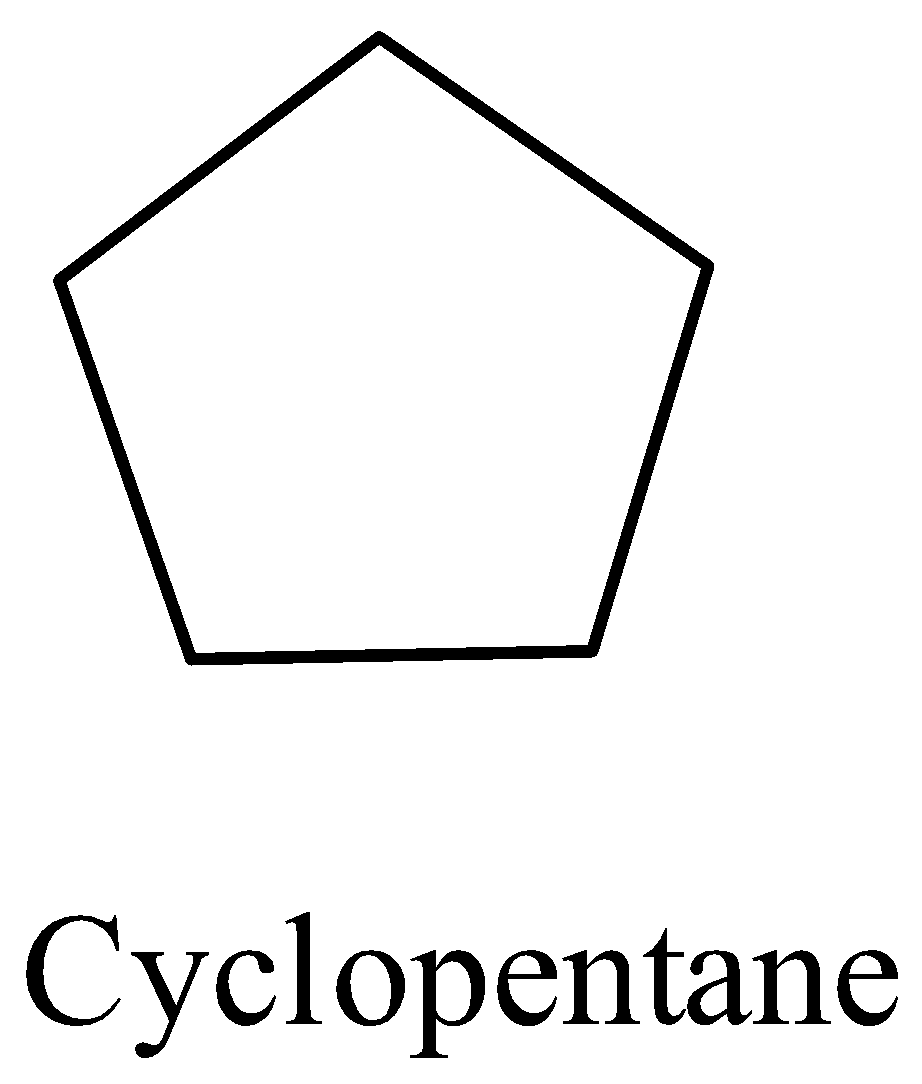
Cyclopentane contains only secondary hydrogens. Then it will undergo chlorination and form only a monochlorinated product.
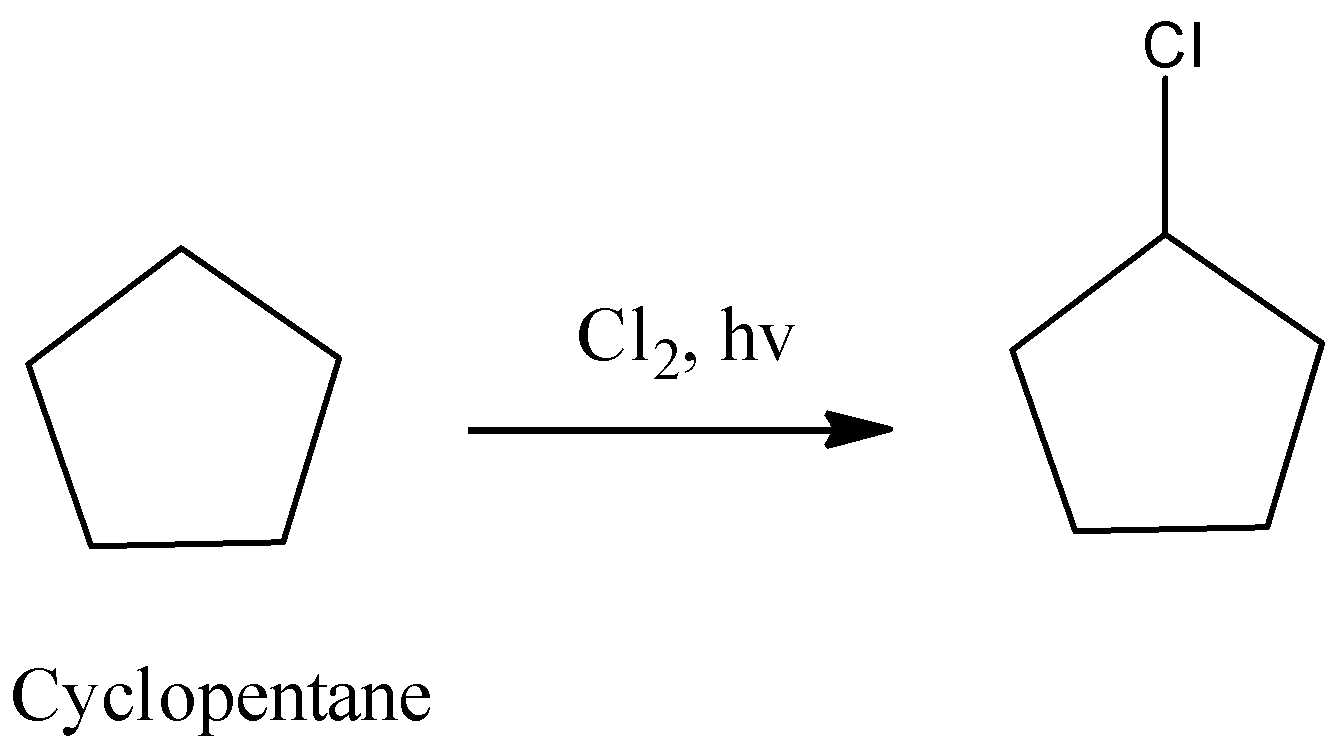
So, the correct answer is “Option D”.
Note: To understand about primary, secondary, and tertiary hydrogens see the below structures of hydrocarbons.

If the product has only secondary hydrogen atoms (means only one type of hydrogen atoms) then monochlorination is possible.
Complete step by step answer:
The given compound has a molecular formula of\[{{C}_{5}}{{H}_{10}}\].
So, the degree of unsaturation of organic compounds can be calculated using \[{{C}_{n}}{{H}_{2n+2}}\] for alkanes, \[{{C}_{n}}{{H}_{2n}}\] for alkenes or cycloalkanes, \[{{C}_{n}}{{H}_{2n-2}}\] for alkynes).
The molecular formula of the given hydrocarbon matches with \[{{C}_{n}}{{H}_{2n}}\] for alkenes or cycloalkanes.
The degree of unsaturation of the given compound is 1. It should have either one double bond or should be a cyclic compound.
To do chlorination or halogenation, the hydrocarbon should have either one double bond or it should be a cyclic compound.
If the hydrocarbon has one double bond, then it will give more than one monochlorinated product. So, the hydrocarbon should be a cyclic compound. Because in cyclic hydrocarbon only one type of hydrocarbons is present (mostly secondary hydrogen atoms are present).
Coming to the given options, option A, 1-pentene (the structure is as follows).

In 1-pentene, primary, secondary hydrogens and one double bond are present.
So, 1-pentene undergoes chlorination and gives a di-chlorinated product. Therefore option A is wrong.

Coming to option B, 2-pentene (the structure is as follows).

In 2-pentene, primary, secondary hydrogens and one double bond are present.
So, 2-pentene undergoes chlorination and gives a di-chlorinated product. Therefore option B is wrong.

Coming to option C, 1-methyl cyclobutane (the structure is as follows).

In 1-methyl cyclobutane, primary, secondary and tertiary hydrogens are present.
So, 1-methyl cyclobutane undergoes chlorination and gives more than one monochlorinated product. Therefore option C is wrong.

Coming to option D, cyclopentane.

Cyclopentane contains only secondary hydrogens. Then it will undergo chlorination and form only a monochlorinated product.

So, the correct answer is “Option D”.
Note: To understand about primary, secondary, and tertiary hydrogens see the below structures of hydrocarbons.




Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




