
Complex, charge of ${\text{Co}}$ is:
A) $ + 2$
B) $ + 3$
C) $ + 1$
D) $ + 4$

Answer
563.4k+ views
Hint: To solve this we must know that the charge on the ${\text{Co}}$ is the oxidation number of ${\text{Co}}$. ${\text{Co}}$ is cobalt which is the central metal atom in the given complex. Remember there are two ${\text{Co}}$ atoms. Four ammine $\left( {{\text{N}}{{\text{H}}_3}} \right)$, two sulphate $\left( {{\text{S}}{{\text{O}}_4}} \right)$, one hydroxide $\left( {{\text{OH}}} \right)$ and one chloride $\left( {{\text{Cl}}} \right)$ ligands are attached to the central metal atom i.e. ${\text{Co}}$.
Complete solution:
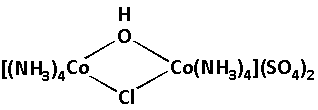
We are given a complex as follows:
In the given complex, we have to calculate the charge of ${\text{Co}}$. The charge on the ${\text{Co}}$ is the oxidation number of ${\text{Co}}$. ${\text{Co}}$ is cobalt which is the central metal atom in the given complex. Also there are two cobalt atoms.
Different ligands are attached to the central cobalt atoms. Four ammine $\left( {{\text{N}}{{\text{H}}_3}} \right)$, two sulphate $\left( {{\text{S}}{{\text{O}}_4}} \right)$, one hydroxide $\left( {{\text{OH}}} \right)$ and one chloride $\left( {{\text{Cl}}} \right)$ ligands are attached to the central cobalt atoms.
Thus,
\[{\text{Charge on the complex}} = 2\left( {{\text{Co}}} \right) + 4\left( {{\text{N}}{{\text{H}}_3}} \right) + 2\left( {{\text{S}}{{\text{O}}_4}} \right) + 1\left( {{\text{OH}}} \right) + 1\left( {{\text{Cl}}} \right)\]
Four ammine $\left( {{\text{N}}{{\text{H}}_3}} \right)$ ligands are attached. The amine ligands are neutral in nature. Thus, the charge on amine ligands is zero.
Two sulphate $\left( {{\text{S}}{{\text{O}}_4}} \right)$ ligands are attached. The sulphate ligands are negative in nature. Each sulphate ligand has a charge of $ - 2$.
One hydroxide $\left( {{\text{OH}}} \right)$ ligand is attached. The hydroxide ligands are negative in nature. Each hydroxide ligand has a charge of $ - 1$.
One chloride $\left( {{\text{Cl}}} \right)$ ligand is attached. The chloride ligands are negative in nature. Each chloride ligand has a charge of $ - 1$.
As there is no charge mentioned on the overall complex we consider that the total charge on the complex is zero.
Let the charge on the central cobalt atom be x. Thus,
\[{\text{0}} = 2\left( x \right) + 4\left( 0 \right) + 2\left( { - 2} \right) + 1\left( { - 1} \right) + 1\left( { - 1} \right)\]
\[{\text{0}} = 2\left( x \right) + 0 - 4 - 1 - 1\]
\[2\left( x \right) = + 4 + 1 + 1\]
\[2\left( x \right) = + 6\]
\[x = + 3\]
Thus, in the complex, charge of ${\text{Co}}$ is \[ + 3\].
Thus, the correct option is (B)
Note:To solve this you must remember that there are two cobalt atoms. If you consider only one cobalt atom then you will get that the charge of ${\text{Co}}$ is \[ + 6\]. Also, remember the charges on each of the ligands attached to the central cobalt atom.
Complete solution:
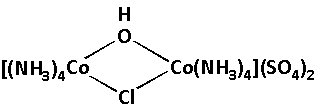
We are given a complex as follows:

In the given complex, we have to calculate the charge of ${\text{Co}}$. The charge on the ${\text{Co}}$ is the oxidation number of ${\text{Co}}$. ${\text{Co}}$ is cobalt which is the central metal atom in the given complex. Also there are two cobalt atoms.
Different ligands are attached to the central cobalt atoms. Four ammine $\left( {{\text{N}}{{\text{H}}_3}} \right)$, two sulphate $\left( {{\text{S}}{{\text{O}}_4}} \right)$, one hydroxide $\left( {{\text{OH}}} \right)$ and one chloride $\left( {{\text{Cl}}} \right)$ ligands are attached to the central cobalt atoms.
Thus,
\[{\text{Charge on the complex}} = 2\left( {{\text{Co}}} \right) + 4\left( {{\text{N}}{{\text{H}}_3}} \right) + 2\left( {{\text{S}}{{\text{O}}_4}} \right) + 1\left( {{\text{OH}}} \right) + 1\left( {{\text{Cl}}} \right)\]
Four ammine $\left( {{\text{N}}{{\text{H}}_3}} \right)$ ligands are attached. The amine ligands are neutral in nature. Thus, the charge on amine ligands is zero.
Two sulphate $\left( {{\text{S}}{{\text{O}}_4}} \right)$ ligands are attached. The sulphate ligands are negative in nature. Each sulphate ligand has a charge of $ - 2$.
One hydroxide $\left( {{\text{OH}}} \right)$ ligand is attached. The hydroxide ligands are negative in nature. Each hydroxide ligand has a charge of $ - 1$.
One chloride $\left( {{\text{Cl}}} \right)$ ligand is attached. The chloride ligands are negative in nature. Each chloride ligand has a charge of $ - 1$.
As there is no charge mentioned on the overall complex we consider that the total charge on the complex is zero.
Let the charge on the central cobalt atom be x. Thus,
\[{\text{0}} = 2\left( x \right) + 4\left( 0 \right) + 2\left( { - 2} \right) + 1\left( { - 1} \right) + 1\left( { - 1} \right)\]
\[{\text{0}} = 2\left( x \right) + 0 - 4 - 1 - 1\]
\[2\left( x \right) = + 4 + 1 + 1\]
\[2\left( x \right) = + 6\]
\[x = + 3\]
Thus, in the complex, charge of ${\text{Co}}$ is \[ + 3\].
Thus, the correct option is (B)
Note:To solve this you must remember that there are two cobalt atoms. If you consider only one cobalt atom then you will get that the charge of ${\text{Co}}$ is \[ + 6\]. Also, remember the charges on each of the ligands attached to the central cobalt atom.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




