
Complete the reaction-
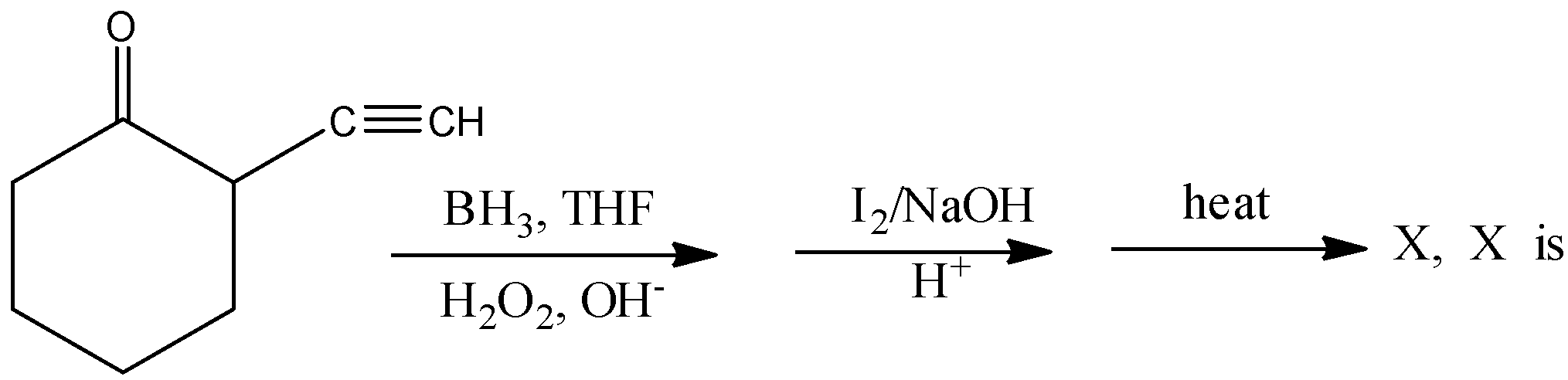
A.
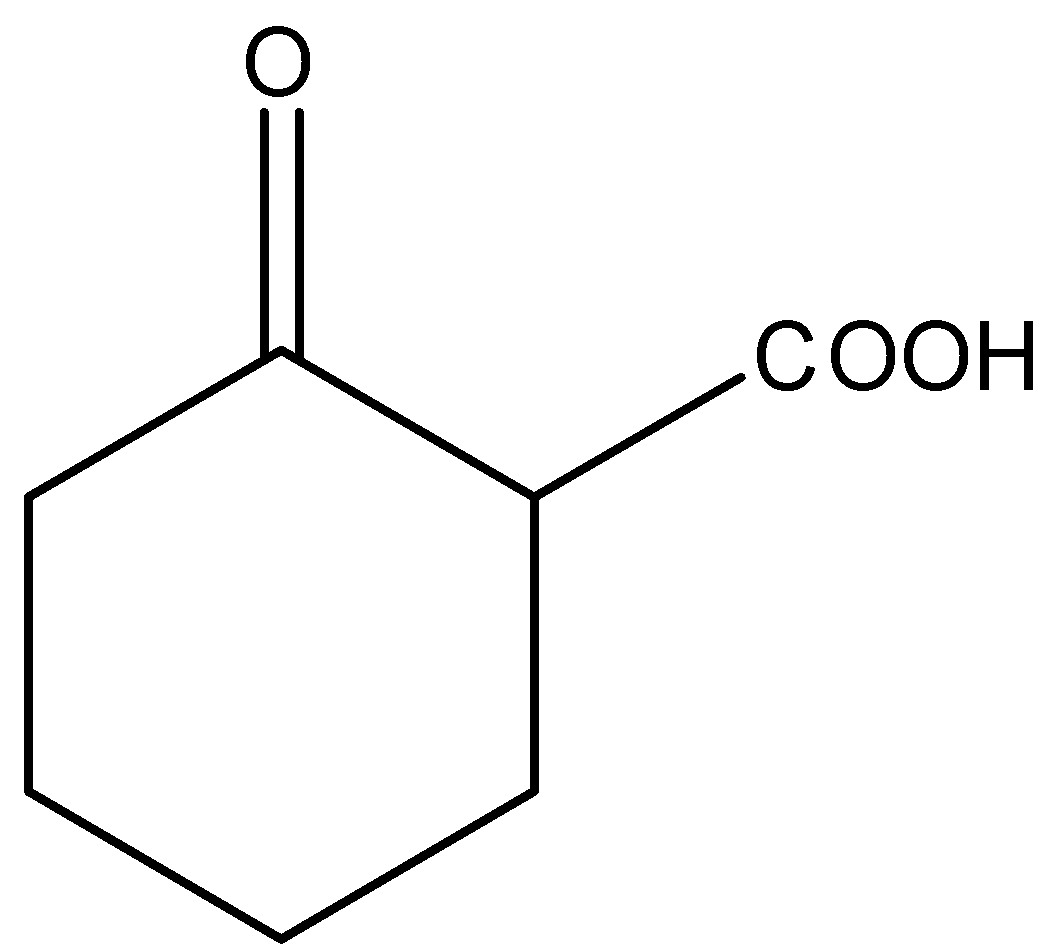
B.
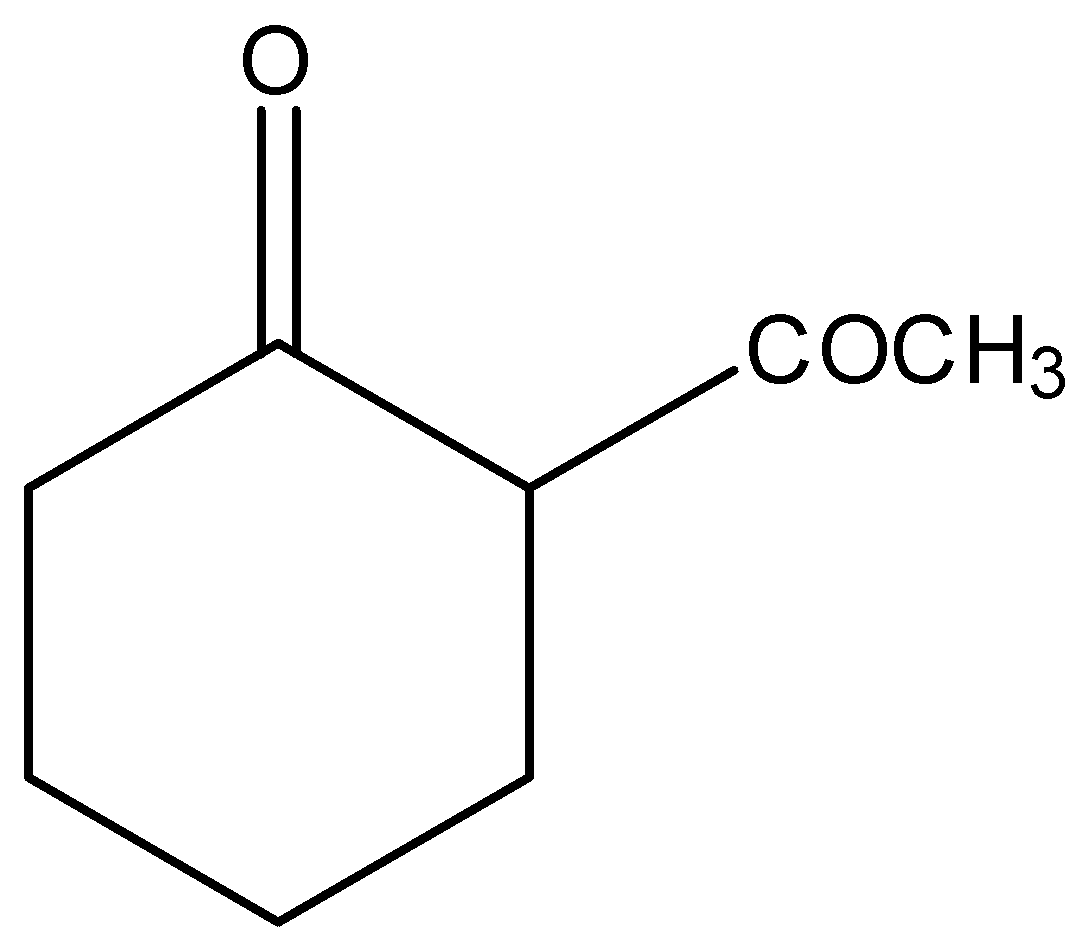
C.
D.





Answer
510.9k+ views
Hint: Here, \[2 - \]ethynylcyclohexan\[ - 1 - \]one is reacted with borane, trihydrofuran, and hydrogen peroxide, there is a formation of one product. And that will react with sodium hydroxide and iodine under heat and there will be the final product. The \[B{H_3}\]reagent is used for hydroboration reaction of alkynes and alkenes. During this reaction, first there is a formation of enol.
Complete answer:
\[2 - \]Oxo cyclohexane\[ - 1 - \]carboxylic acid is not formed by this reaction. Hence, option (A) is incorrect.
There will not be a formation of \[2 - \]Acetylcyclohexan\[ - 1 - \]one. Hence, option (B) is correct.
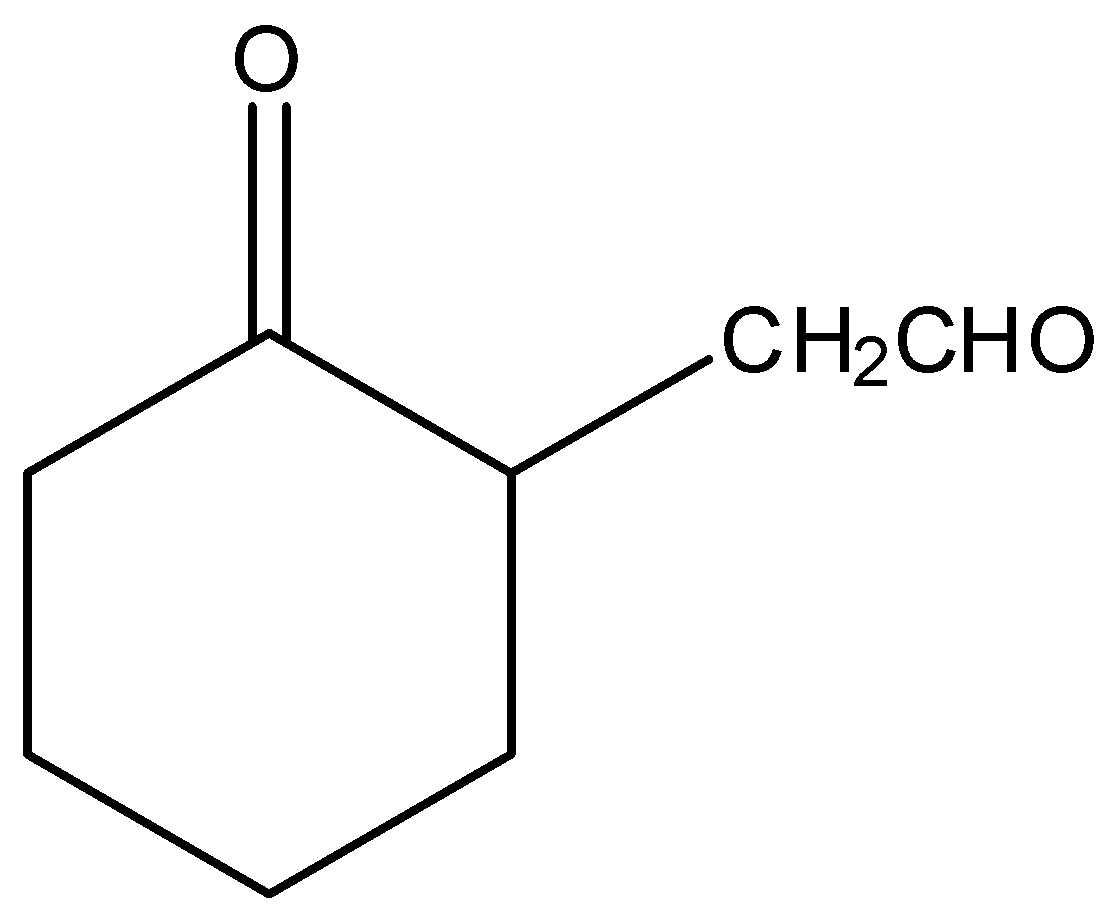
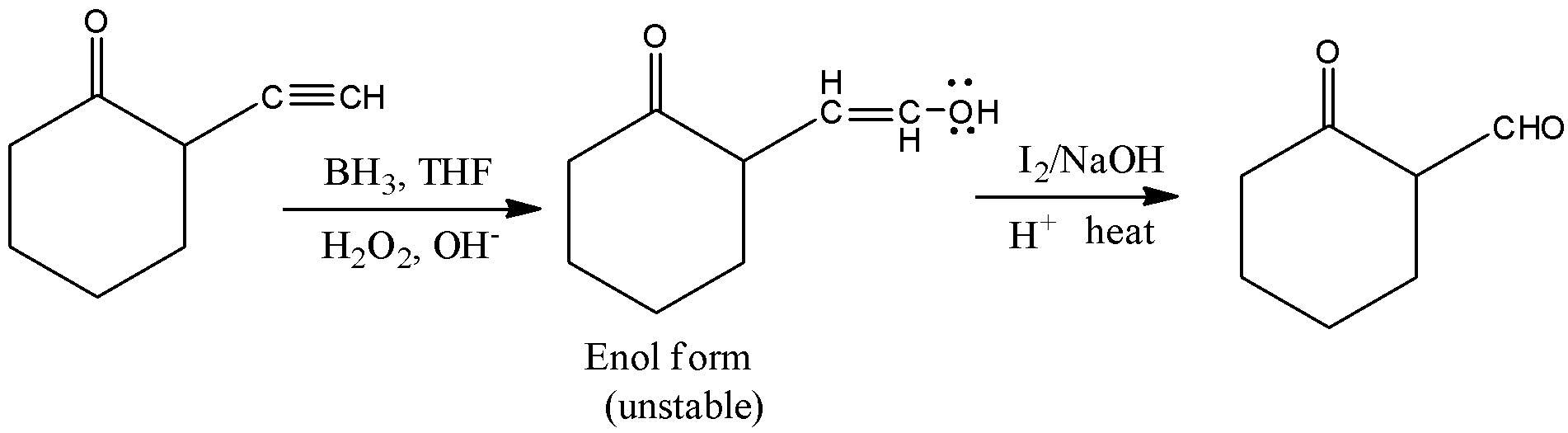
Here, \[2 - \]ethynylcyclohexan\[ - 1 - \]one is reacted with borane in the presence of trihydrofuran, (THF) and hydrogen peroxide and there is a formation of unstable enol form. The borane is mainly used for the hydroboration of alkynes. Thus, by hydroboration of alkynes, there is a formation of a product called enol which is highly unstable. Therefore, it will react with sodium hydroxide and in the presence of heat, there is a formation of \[2 - \](\[2 - \]oxocyclohexyl) acetaldehyde. Let’s see the reaction,
Hence, option (C) is correct.
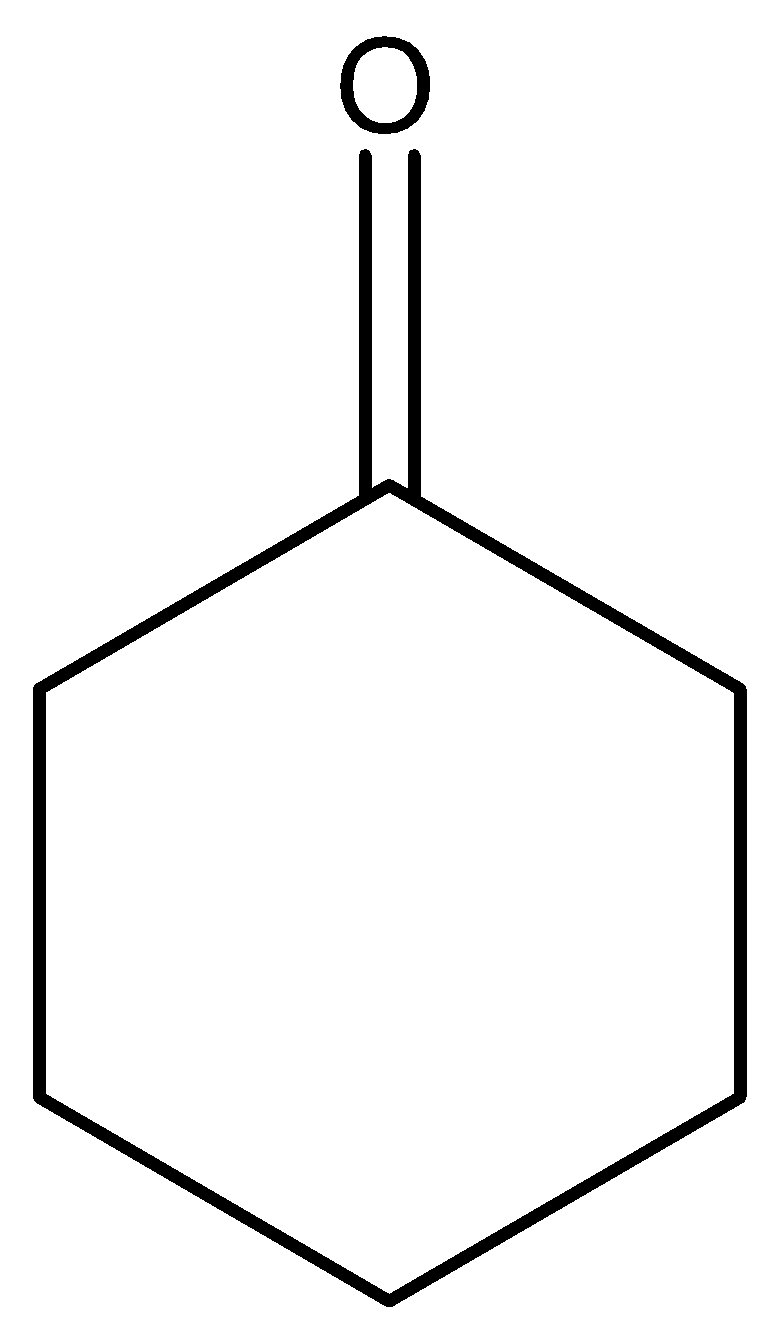
There will not be a formation of cyclohexanone as the final product. Hence, the option (D) is incorrect.
Hence, option (C) is correct.
Note:
By the hydroboration of alkynes there is a formation of product called enol and this process is known as tautomerization. And this enol is converted into keto form which is more stable aldehyde. The enol is otherwise called, half alcohol. And the constitutional isomer is aldehyde. This type of reaction is called keto – enol tautomerism.
Complete answer:
\[2 - \]Oxo cyclohexane\[ - 1 - \]carboxylic acid is not formed by this reaction. Hence, option (A) is incorrect.
There will not be a formation of \[2 - \]Acetylcyclohexan\[ - 1 - \]one. Hence, option (B) is correct.
Here, \[2 - \]ethynylcyclohexan\[ - 1 - \]one is reacted with borane in the presence of trihydrofuran, (THF) and hydrogen peroxide and there is a formation of unstable enol form. The borane is mainly used for the hydroboration of alkynes. Thus, by hydroboration of alkynes, there is a formation of a product called enol which is highly unstable. Therefore, it will react with sodium hydroxide and in the presence of heat, there is a formation of \[2 - \](\[2 - \]oxocyclohexyl) acetaldehyde. Let’s see the reaction,

Hence, option (C) is correct.
There will not be a formation of cyclohexanone as the final product. Hence, the option (D) is incorrect.
Hence, option (C) is correct.
Note:
By the hydroboration of alkynes there is a formation of product called enol and this process is known as tautomerization. And this enol is converted into keto form which is more stable aldehyde. The enol is otherwise called, half alcohol. And the constitutional isomer is aldehyde. This type of reaction is called keto – enol tautomerism.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




