
Complete the following reactions.
(a)
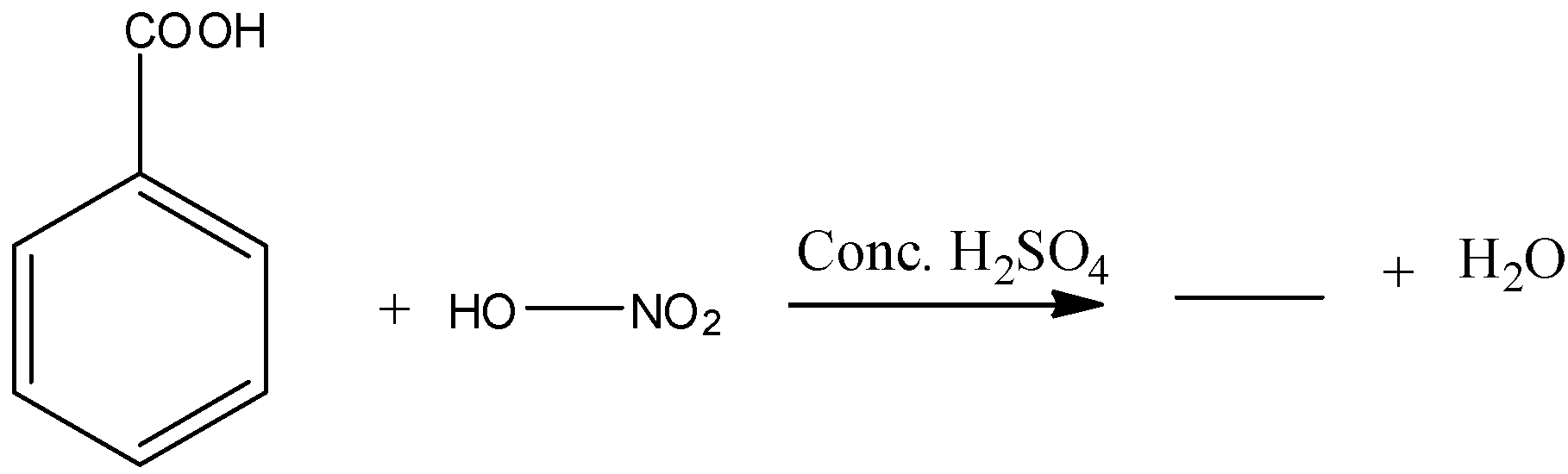
(b)
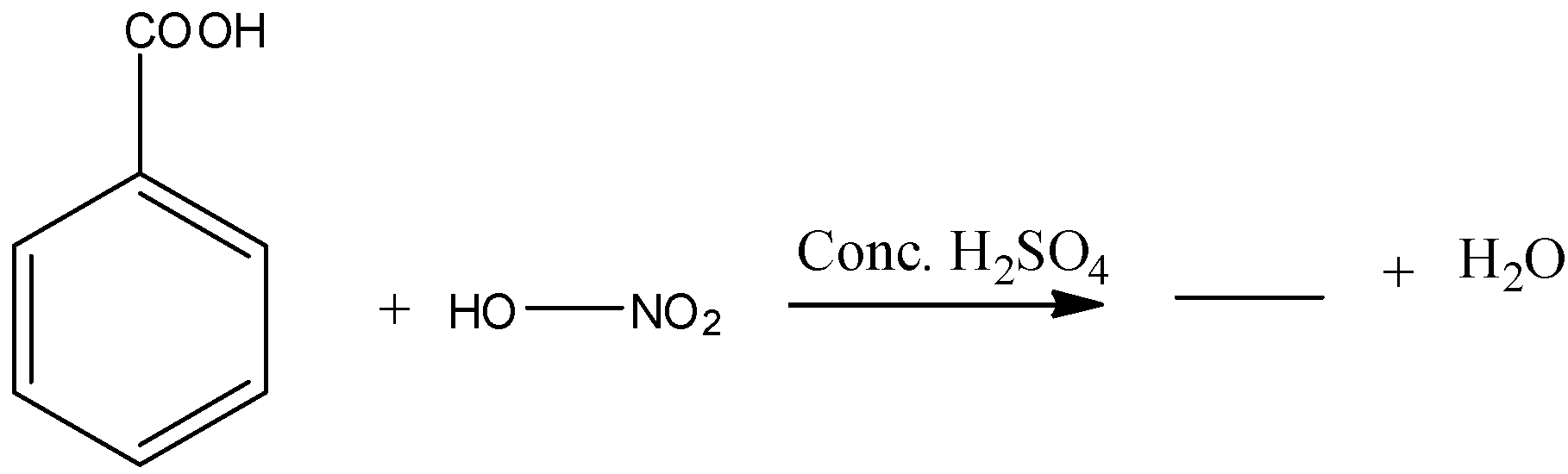

Answer
574.5k+ views
Hint: The presence of the electron withdrawing group directs the reactants to meta position that is why electron withdrawing groups are called meta directing groups. Carboxylic acid comes under electron withdrawing groups and acts as a meta directing group.
Complete step by step answer:
- In the question it is given to complete the chemical reactions.
a) The given chemical reaction is as follows.
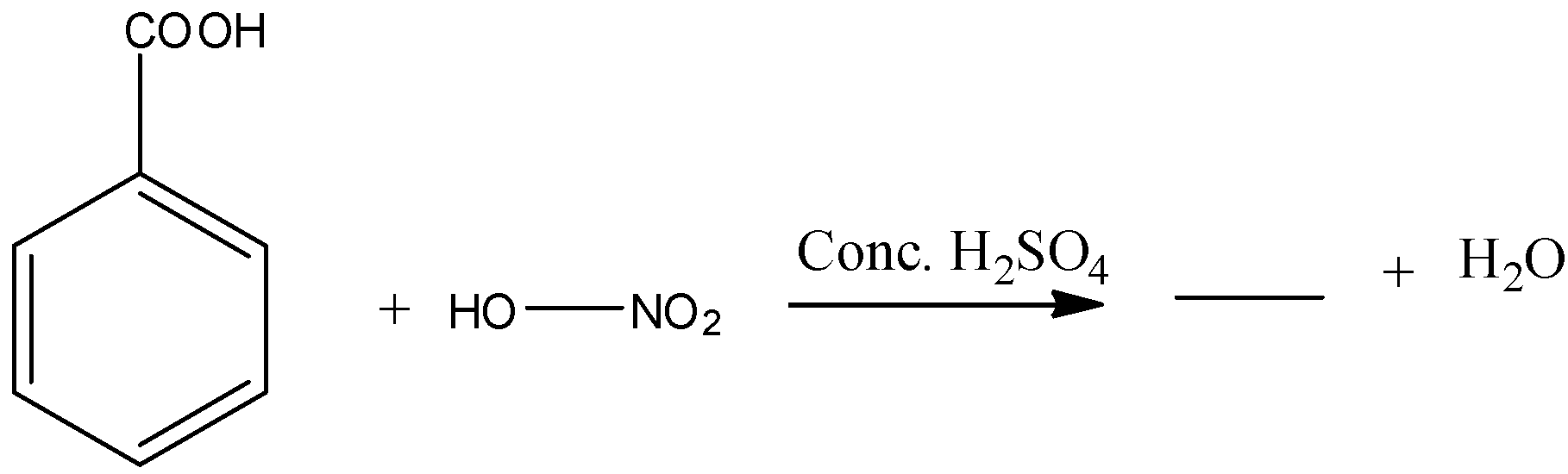
- In the above chemical reaction benzoic acid reacts with nitric acid in presence of sulphuric acid.
- The chemical reaction of benzoic acid reacts with nitric acid in presence of sulphuric acid is as follows.
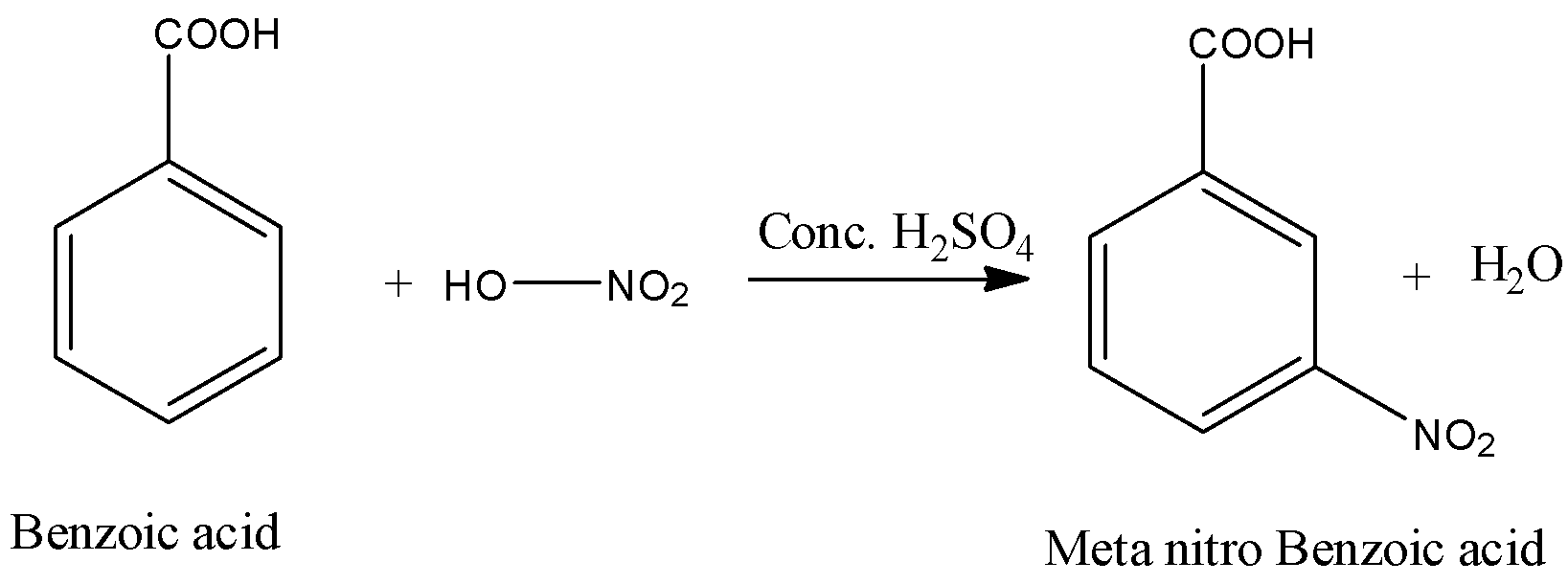
- In the above chemical reaction nitric acid reacts with benzoic acid and forms meta nitro benzoic acid and water as the products.
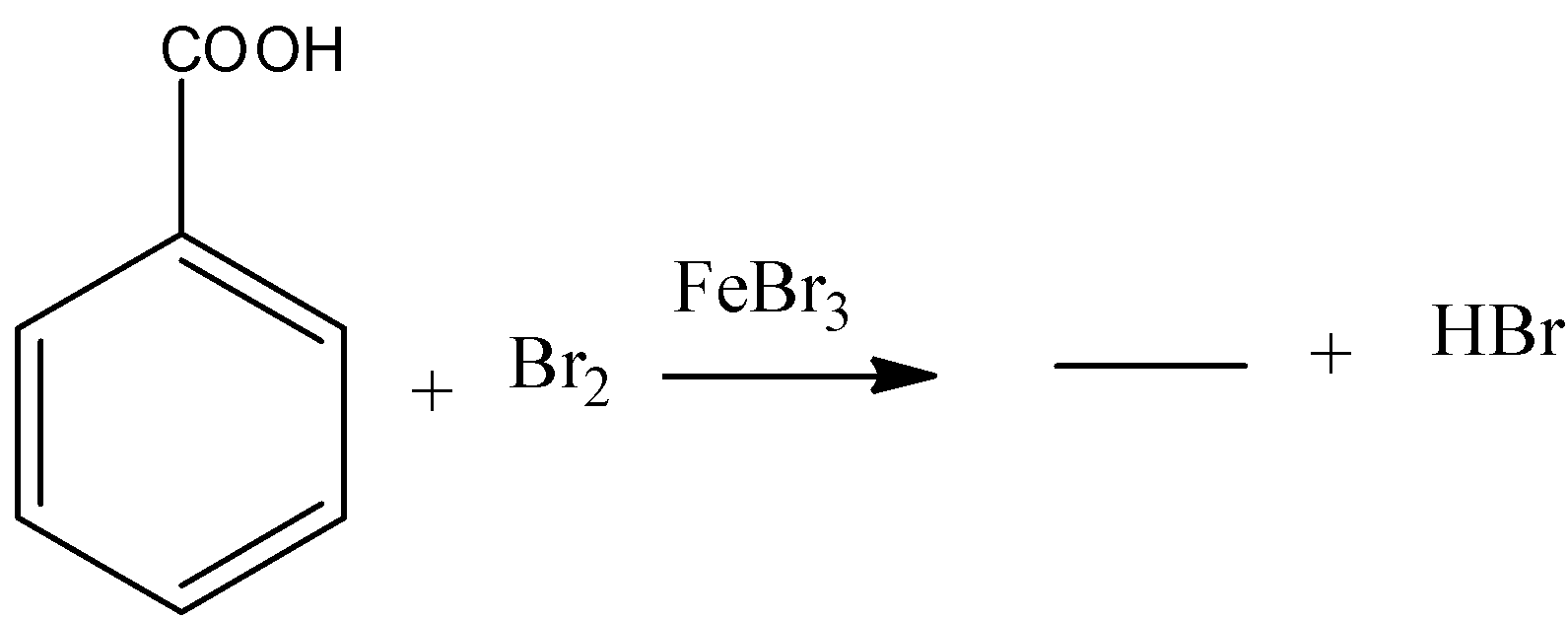
b) The given chemical reaction is as follows.
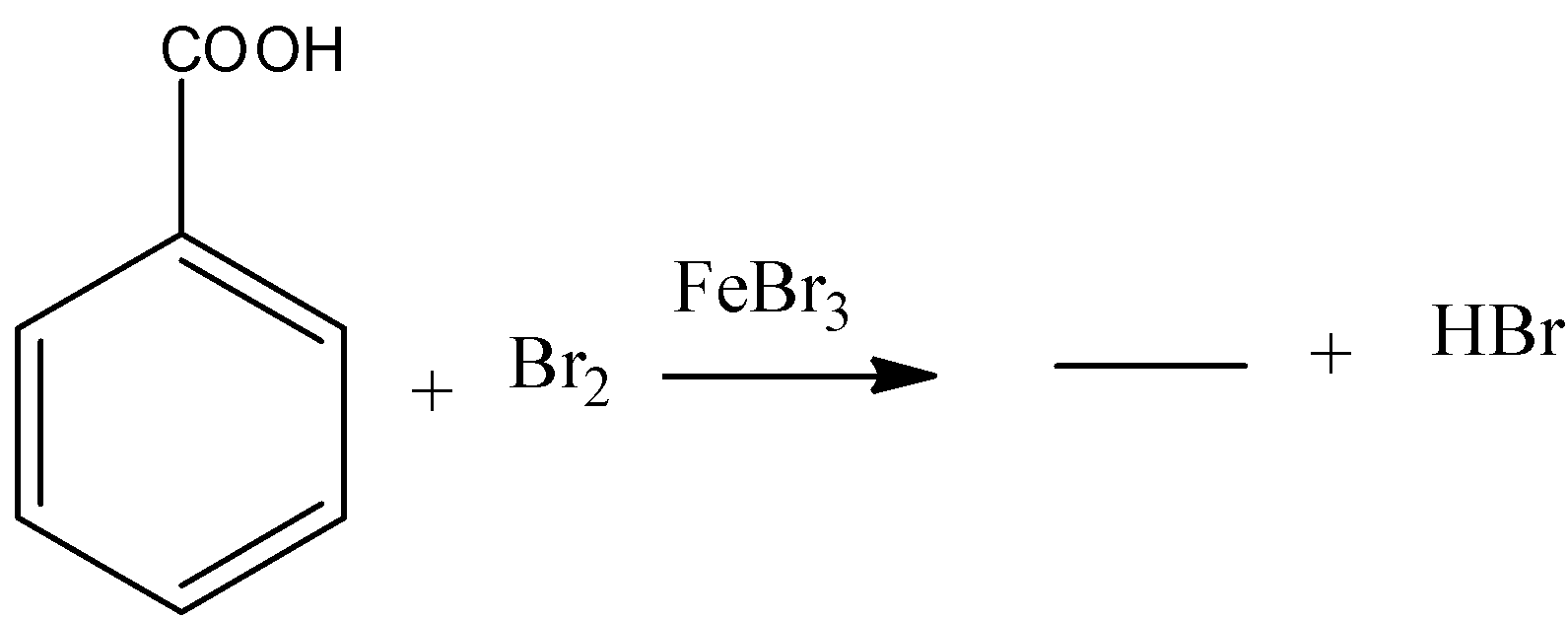
- In the above chemical reaction benzoic acid reacts with bromine in presence of ferric bromide reagent.
- The chemical reaction of benzoic acid reacts with bromine in presence of ferric bromide is as follows
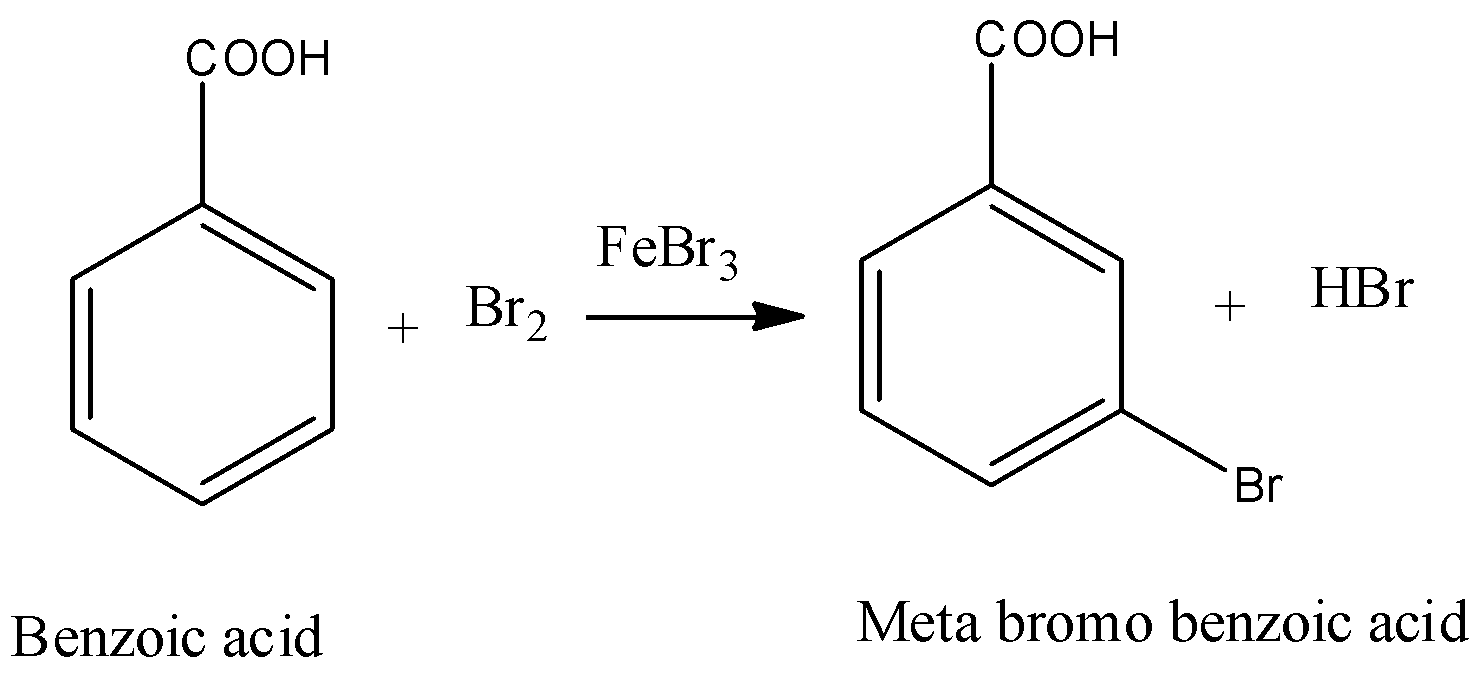
- In the above chemical reaction bromine reacts with benzoic acid and forms meta bromo benzoic acid and hydrogen bromide as the products.
Note: If we are going to add a nitro functional group in the benzene then it is called nitration. If we are going to add bromine in the benzene ring then it is called bromination or halogenation. In presence of the electron withdrawing group in benzene nitration and bromination occurs at meta position in the benzene ring.
Complete step by step answer:
- In the question it is given to complete the chemical reactions.
a) The given chemical reaction is as follows.
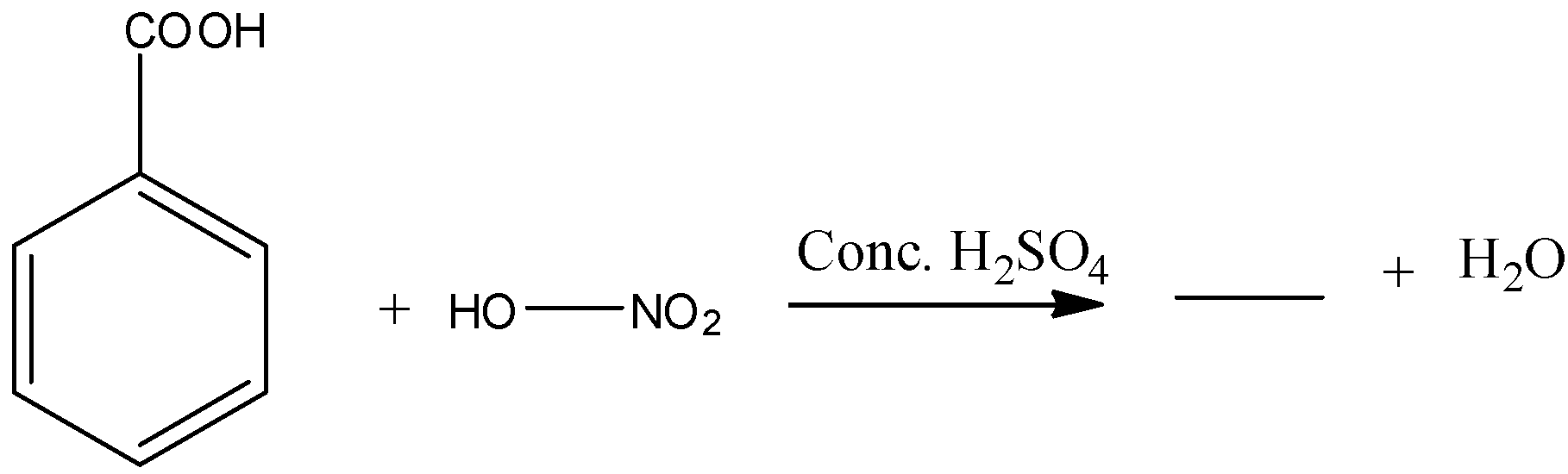
- In the above chemical reaction benzoic acid reacts with nitric acid in presence of sulphuric acid.
- The chemical reaction of benzoic acid reacts with nitric acid in presence of sulphuric acid is as follows.

- In the above chemical reaction nitric acid reacts with benzoic acid and forms meta nitro benzoic acid and water as the products.
b) The given chemical reaction is as follows.

- In the above chemical reaction benzoic acid reacts with bromine in presence of ferric bromide reagent.
- The chemical reaction of benzoic acid reacts with bromine in presence of ferric bromide is as follows

- In the above chemical reaction bromine reacts with benzoic acid and forms meta bromo benzoic acid and hydrogen bromide as the products.
Note: If we are going to add a nitro functional group in the benzene then it is called nitration. If we are going to add bromine in the benzene ring then it is called bromination or halogenation. In presence of the electron withdrawing group in benzene nitration and bromination occurs at meta position in the benzene ring.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




