
compare the bond length in the given compounds across (carbon-oxygen):
A.
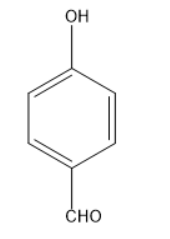
B.
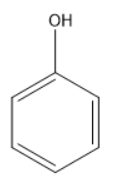
C.
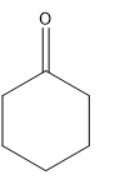



Answer
539.7k+ views
Hint: bond length is defined as the measure of the distance which is calculated between the nuclei of two atoms that are bonded chemically. The approximate value for bond length is the sum of the covalent radii of the two bonded atoms. The relationship between bond order and bond length is inverse; that means higher is the bond order, shorter will be the bond length.
Complete step by step answer:
In the following given compounds the highest bond length is of ‘c’ because it contains a double bonded oxygen atom which is attached to a cyclohexane ring. The phenol which is ‘b’ has higher bond length than ‘a’ because it has a partial double bond character. It contains resonating structure due to resonance effect and forms a phenoxide ion. The lone pair of the oxygen of hydroxyl group will be in conjugation with the phenyl ring and attain partial double bond character. While in structure ‘a’ we have least bond length as it has high bond order and no double bond character is present in it and it shows no resonance effect. So the order of the bond length will be the following
\[a < b < c\]
Note: the bond length of the element is directly proportional to the radii of the atom. So the bond length of the elements decreases across the period and decreases down the group. The bond parameters can be determined by following techniques and they are rotational spectroscopy, X-ray diffraction and neutron diffraction.
Complete step by step answer:
In the following given compounds the highest bond length is of ‘c’ because it contains a double bonded oxygen atom which is attached to a cyclohexane ring. The phenol which is ‘b’ has higher bond length than ‘a’ because it has a partial double bond character. It contains resonating structure due to resonance effect and forms a phenoxide ion. The lone pair of the oxygen of hydroxyl group will be in conjugation with the phenyl ring and attain partial double bond character. While in structure ‘a’ we have least bond length as it has high bond order and no double bond character is present in it and it shows no resonance effect. So the order of the bond length will be the following
\[a < b < c\]
Note: the bond length of the element is directly proportional to the radii of the atom. So the bond length of the elements decreases across the period and decreases down the group. The bond parameters can be determined by following techniques and they are rotational spectroscopy, X-ray diffraction and neutron diffraction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




