
Compare Resonance Energy why?

Answer
588.6k+ views
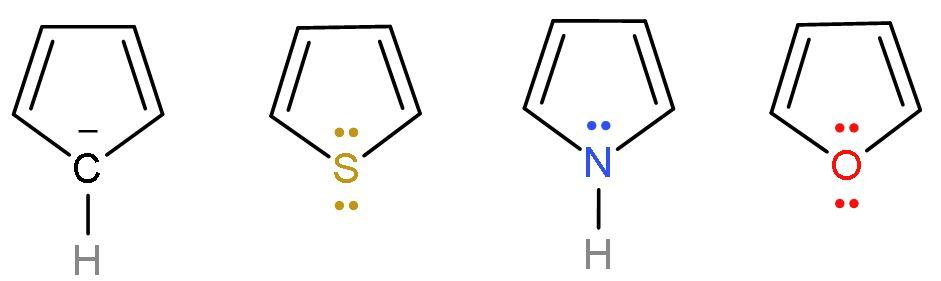
Hint: The resonance energy is dependent on the stability and aromaticity of the compound. Resonance energy $\propto $ stability of the compound $\propto $ aromaticity. The main concept here, to differentiate the compounds is the electronegativity of the atoms attached like oxygen, carbon, sulphur and nitrogen. All the compounds are isoelectronic in terms of $\pi -$ electrons.
Complete step by step answer:
Let us first discuss the features of the compounds and then, we will see the concept to differentiate the four compounds.
All the above compounds are aromatic. So, stability will be seen through a new concept. Here, the concept is based on the ‘electronegativity of the elements’ present in the compounds.
-The electronegativity will play a role here, as the electronegativity of an element will pull the electron cloud towards itself. Thus, there will be an uneven charge distribution and less likely to undergo resonance. More the electronegativity of the hetero atoms, less will be the aromaticity of the compound. We know that oxygen is the most electronegative atom among all. The order of electronegativity is $\text{O}>\text{N}>\text{S}>\text{C}$.
So, it is clear that furan is least and cyclopentadienyl anion is the most aromatic compound. The order of aromaticity and resonance energy will be cyclopentadienyl anion$ > $thiophene$ > $pyrrole$ > $furan.
Note: One more factor comes into play here, that is bond length. The bond length of the element with carbon will increase in the order, $\left( \text{C}-\text{S} \right)>\left( \text{C}-\text{O} \right)>\left( \text{C}-\text{N} \right)>\left( \text{C}-\text{C} \right)$. As the bond length increases, the bonding or overlapping of elements will become difficult. Thus, shows more reactivity.
Complete step by step answer:
Let us first discuss the features of the compounds and then, we will see the concept to differentiate the four compounds.
| S. No. | Structures | Name of the compound | Features |
| 1. | 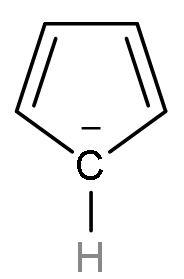
| Cyclopentadienyl anion | It is a homocyclic (made of the same atom only) compound which is made of carbon atoms. It is an aromatic compound as it follows Huckel’s rule or (4n+2) $\pi $-electrons. Because it has 6 $\pi $ electrons, two from negative charge and four from $\left( \text{C}-\text{C} \right)$ double bond. |
| 2. | 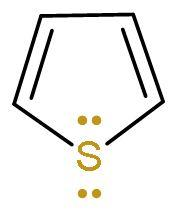
| Thiophene | It is a heterocyclic (made of different atoms) compound which is made of carbon atom and sulphur atom. It is an aromatic compound as it follows Huckel’s rule or (4n+2) $\pi $-electrons. Because it has 6 $\pi $ electrons, two from lone pairs of sulphur and four from $\left( \text{C}-\text{C} \right)$ double bond. |
| 3. | 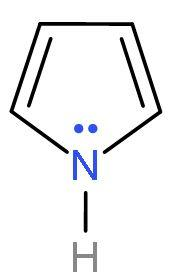
| Pyrrole | It is a heterocyclic (made of different atoms) compound which is made of carbon atom and nitrogen atom. It is an aromatic compound as it follows Huckel’s rule or (4n+2) $\pi $-electrons. Because it has 6 $\pi $ electrons, two from lone pairs of nitrogen and four from $\left( \text{C}-\text{C} \right)$ double bond. |
| 4. | 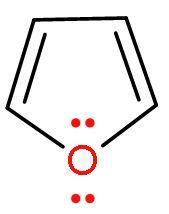
| Furan | It is a heterocyclic (made of different atoms) compound which is made of carbon atom and oxygen atom. It is an aromatic compound as it follows Huckel’s rule or (4n+2) $\pi $-electrons. Because it has 6 $\pi $ electrons, two from lone pair of oxygen and four from $\left( \text{C}-\text{C} \right)$ double bond. It looks similar to Thiophene. |
All the above compounds are aromatic. So, stability will be seen through a new concept. Here, the concept is based on the ‘electronegativity of the elements’ present in the compounds.
-The electronegativity will play a role here, as the electronegativity of an element will pull the electron cloud towards itself. Thus, there will be an uneven charge distribution and less likely to undergo resonance. More the electronegativity of the hetero atoms, less will be the aromaticity of the compound. We know that oxygen is the most electronegative atom among all. The order of electronegativity is $\text{O}>\text{N}>\text{S}>\text{C}$.
So, it is clear that furan is least and cyclopentadienyl anion is the most aromatic compound. The order of aromaticity and resonance energy will be cyclopentadienyl anion$ > $thiophene$ > $pyrrole$ > $furan.
Note: One more factor comes into play here, that is bond length. The bond length of the element with carbon will increase in the order, $\left( \text{C}-\text{S} \right)>\left( \text{C}-\text{O} \right)>\left( \text{C}-\text{N} \right)>\left( \text{C}-\text{C} \right)$. As the bond length increases, the bonding or overlapping of elements will become difficult. Thus, shows more reactivity.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




