
What is the combining ratio of glycerol and fatty acids when they combine to form triglyceride?
A) $3:4$
B) $3:2$
C) $1:3$
D) $1:2$
Answer
591.6k+ views
Hint: The reaction of combining glycerol and fatty acids to form triglycerides is called an esterification reaction. In the reaction of esterification, one fatty acid molecule esterifies the one alcohol group. One can use this data and can analyze the reaction in order to find the ratio of glycerol and fatty acids.
Complete step by step answer:
1) The triglycerides are formed by the reaction of glycerol and fatty acids, this reaction is called esterification reaction.
2) The fatty acids are the organic compounds that are represented by the general formula $C{H_3}{\left( {C{H_2}} \right)_n}COOH$ in which the $n$ stand for the range between $2$ to $28$ and always has an even number.
3) Triglyceride formation reaction can be written as follows,
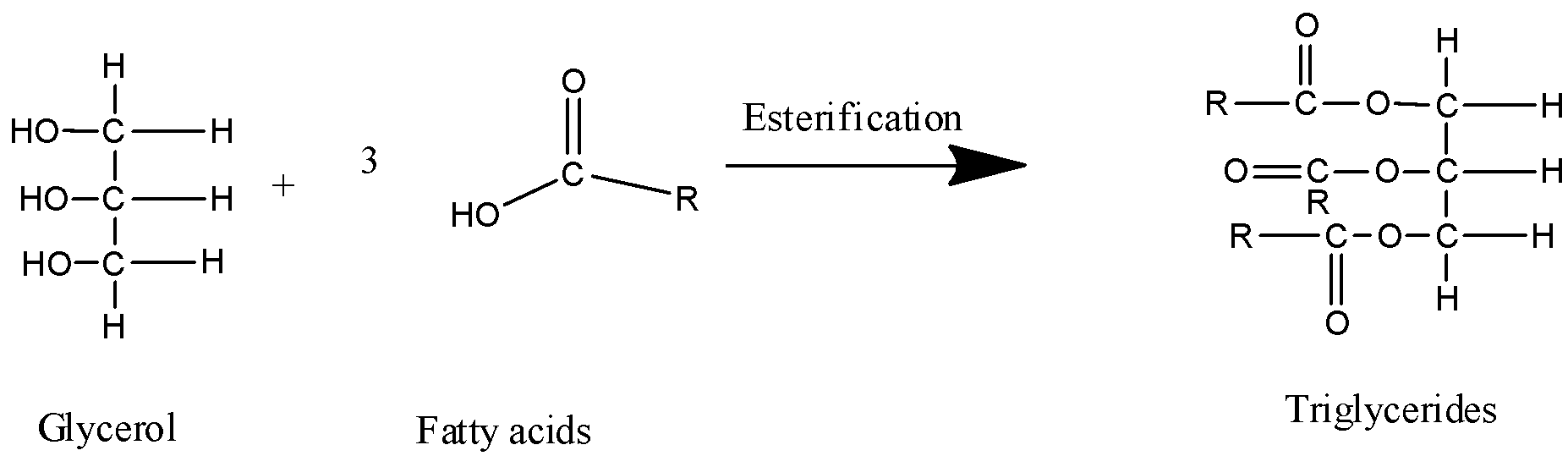
In the above reaction glycerol is a triol i.e. it has three hydroxyl functional groups in its structure. The fatty acid is a compound that has a long-chain having $12$ carbon atoms to $24$ carbon atoms in length which has a carboxyl functional group attached to it. Each fatty acid molecule of the three molecules undergoes an esterification reaction with one of the hydroxyl groups of the glycerol molecule and esterifies all the three hydroxyl groups in the structure. So, overall there are three molecules of fatty acids required for esterification of the one glycerol molecule.
Therefore, we have come to the conclusion that the combining ratio of glycerol and fatty acids when they combine to form triglyceride is $1:3$ which shows the option C as a correct choice.
Note:
While the formation of triglycerides we can use three different types of fatty acids (which in the above case we used three molecules of only one form of fatty acid) to react with glycerol and form different triglycerides. Triglycerides are used by the human body as the long term storage form of energy. Triglycerides are also called as triesters of glycerol.
Complete step by step answer:
1) The triglycerides are formed by the reaction of glycerol and fatty acids, this reaction is called esterification reaction.
2) The fatty acids are the organic compounds that are represented by the general formula $C{H_3}{\left( {C{H_2}} \right)_n}COOH$ in which the $n$ stand for the range between $2$ to $28$ and always has an even number.
3) Triglyceride formation reaction can be written as follows,

In the above reaction glycerol is a triol i.e. it has three hydroxyl functional groups in its structure. The fatty acid is a compound that has a long-chain having $12$ carbon atoms to $24$ carbon atoms in length which has a carboxyl functional group attached to it. Each fatty acid molecule of the three molecules undergoes an esterification reaction with one of the hydroxyl groups of the glycerol molecule and esterifies all the three hydroxyl groups in the structure. So, overall there are three molecules of fatty acids required for esterification of the one glycerol molecule.
Therefore, we have come to the conclusion that the combining ratio of glycerol and fatty acids when they combine to form triglyceride is $1:3$ which shows the option C as a correct choice.
Note:
While the formation of triglycerides we can use three different types of fatty acids (which in the above case we used three molecules of only one form of fatty acid) to react with glycerol and form different triglycerides. Triglycerides are used by the human body as the long term storage form of energy. Triglycerides are also called as triesters of glycerol.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




