
Choose the correct option for - which is not the structure of $ {C_4}{H_9}Br $
(A) $ 1 - $ Bromobutane
(B) $ 2 - $ Bromobutane
(C) $ 1 - $ Bromo - $ 2 - $ methylpropane
(D) Isobutane
Answer
528.9k+ views
Hint :Bromobutane is a colourless liquid, but its impure sample can be seen as slightly yellow in colour. Bromobutane is an organobromine compound with a molecular formula of $ {C_4}{H_9}Br $ and the boiling point of around $ 100 - {205^o}C $ .
Complete Step By Step Answer:
To solve this question we should have some insight regarding the conformation of structures.
Looking at our options - $ 1 - $ Bromobutane and $ 2 - $ Bromobutane, let’s have a look at their structure for the better understanding –
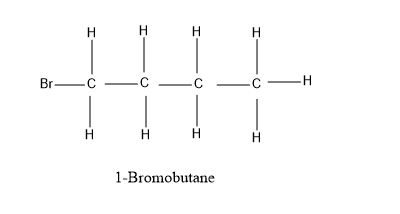
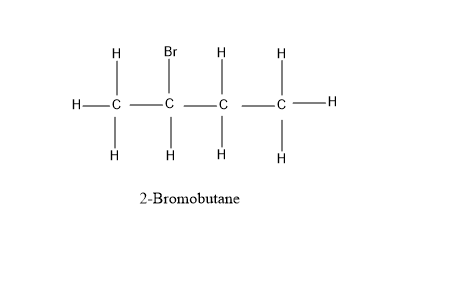
As, it is evident from the diagram above, that $ 1 - $ Bromobutane and $ 2 - $ Bromobutane fits completely into the formula $ {C_4}{H_9}Br $ . All the valencies of all the atoms are completely satisfied and have a stable structure under this formula - $ {C_4}{H_9}Br $ .
Similarly. $ 1 - $ Bromo - $ 2 - $ methyl propane – also has a stable structure satisfying all the valences of each atom.
But, coming to our option D , it is isobutane . It has a molecular formula of $ {C_4}{H_{10}} $ . Isobutane does not consist of bromine atoms. So it does not fit in our given formula $ {C_4}{H_9}Br $ .
So, the correct option to our question is D i.e. isobutane.
Note :
For $ {C_4}{H_9}Br $ we have four possible structural isomers and they are - $ 1 - $ Bromobutane and $ 2 - $ Bromobutane, $ 1 - $ Bromo - $ 2 - $ methylpropane, these three we just read above, but the fourth isomer that is possible is – tert-butyl bromide or $ 2 - $ Bromo - $ 2 - $ methylpropane. These bromobutane are less soluble in water and are denser than water.
Complete Step By Step Answer:
To solve this question we should have some insight regarding the conformation of structures.
Looking at our options - $ 1 - $ Bromobutane and $ 2 - $ Bromobutane, let’s have a look at their structure for the better understanding –


As, it is evident from the diagram above, that $ 1 - $ Bromobutane and $ 2 - $ Bromobutane fits completely into the formula $ {C_4}{H_9}Br $ . All the valencies of all the atoms are completely satisfied and have a stable structure under this formula - $ {C_4}{H_9}Br $ .
Similarly. $ 1 - $ Bromo - $ 2 - $ methyl propane – also has a stable structure satisfying all the valences of each atom.
But, coming to our option D , it is isobutane . It has a molecular formula of $ {C_4}{H_{10}} $ . Isobutane does not consist of bromine atoms. So it does not fit in our given formula $ {C_4}{H_9}Br $ .
So, the correct option to our question is D i.e. isobutane.
Note :
For $ {C_4}{H_9}Br $ we have four possible structural isomers and they are - $ 1 - $ Bromobutane and $ 2 - $ Bromobutane, $ 1 - $ Bromo - $ 2 - $ methylpropane, these three we just read above, but the fourth isomer that is possible is – tert-butyl bromide or $ 2 - $ Bromo - $ 2 - $ methylpropane. These bromobutane are less soluble in water and are denser than water.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




