
How chlorobenzene is obtained from aniline?
Answer
536.1k+ views
Hint: We know that we can prepare chlorobenzene from aniline by using Sand Meyer reaction, by using a fitting reaction we can prepare diphenyl from chlorobenzene. Diazotization is the reaction of a substituted aromatic amine with nitrous acid to form an aromatic diazonium salt. Usually, sodium nitrate and hydrochloric acid react at $ 0\text{ }{}^\circ C\text{ }to\text{ }5\text{ }{}^\circ C $ to generate the nitrous acid.
Complete step by step solution:
Sandmeyer reaction: “The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts. It is an example of a radical-nucleophilic aromatic substitution”. It is a two-step reaction in the first step aniline is going to convert to benzene diazonium chloride using sodium Nitrate and aqueous hydrochloric acid.
The Sandmeyer reaction: The Sandmeyer reaction is the $ CuX $ -catalyzed reaction of an aromatic diazonium salt with a halide ion to form an aromatic halide.
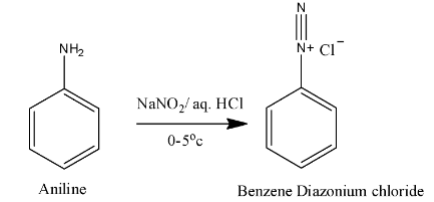
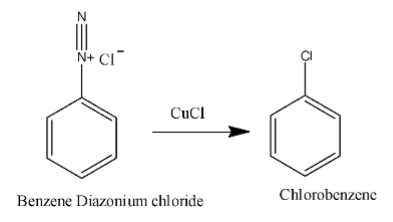
In the second step benzene diazonium chloride reacts with cuprous chloride and forms chloro benzene. Aryl diazonium chloride is very reactive; whenever aryl diazonium chloride forms it will react with cuprous chloride and forms chlorobenzene.
Fittig reaction: “Fittig reaction is the chemical reaction of aryl halides with sodium metal in the presence of dry ether to give substituted aromatic compounds”. Two moles of chlorobenzene react in presence of sodium and dry ether and forms biphenyl compounds and two moles of sodium chloride (insoluble in dry ether)
Note:
Remember that don’t be confused with Sandmeyer reaction and Fittig reaction Sandmeyer reaction: Preparation of chlorobenzene from Aniline by using sodium nitrate, aq. Hydrochloric acid and cuprous chloride. Fittig reaction: Preparation of Diphenyl form Chlorobenzene by using sodium metal and dry ether.
Complete step by step solution:
Sandmeyer reaction: “The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts. It is an example of a radical-nucleophilic aromatic substitution”. It is a two-step reaction in the first step aniline is going to convert to benzene diazonium chloride using sodium Nitrate and aqueous hydrochloric acid.
The Sandmeyer reaction: The Sandmeyer reaction is the $ CuX $ -catalyzed reaction of an aromatic diazonium salt with a halide ion to form an aromatic halide.

In the second step benzene diazonium chloride reacts with cuprous chloride and forms chloro benzene. Aryl diazonium chloride is very reactive; whenever aryl diazonium chloride forms it will react with cuprous chloride and forms chlorobenzene.

Fittig reaction: “Fittig reaction is the chemical reaction of aryl halides with sodium metal in the presence of dry ether to give substituted aromatic compounds”. Two moles of chlorobenzene react in presence of sodium and dry ether and forms biphenyl compounds and two moles of sodium chloride (insoluble in dry ether)
Note:
Remember that don’t be confused with Sandmeyer reaction and Fittig reaction Sandmeyer reaction: Preparation of chlorobenzene from Aniline by using sodium nitrate, aq. Hydrochloric acid and cuprous chloride. Fittig reaction: Preparation of Diphenyl form Chlorobenzene by using sodium metal and dry ether.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




