
How many chiral carbon atoms are there in the open-chain form of glucose?
Answer
558.3k+ views
Hint: The chiral carbon atom is the carbon that is attached to four different atoms or groups. The structure of glucose has one aldehyde group at any end of the carbon chain in the open-chain form of glucose. One can try to draw the structure and decide the chiral centers based on atoms attached to the carbon atom.
Complete step by step answer:
1) First of all, we will try to understand the meaning of the chiral carbon atom. A chiral carbon atom must be attached to four different atoms or groups. If there are two or more than two same atoms or groups attached then it will not be a chiral carbon.
2) Now let us first see the structure of the open chain form of glucose as below,
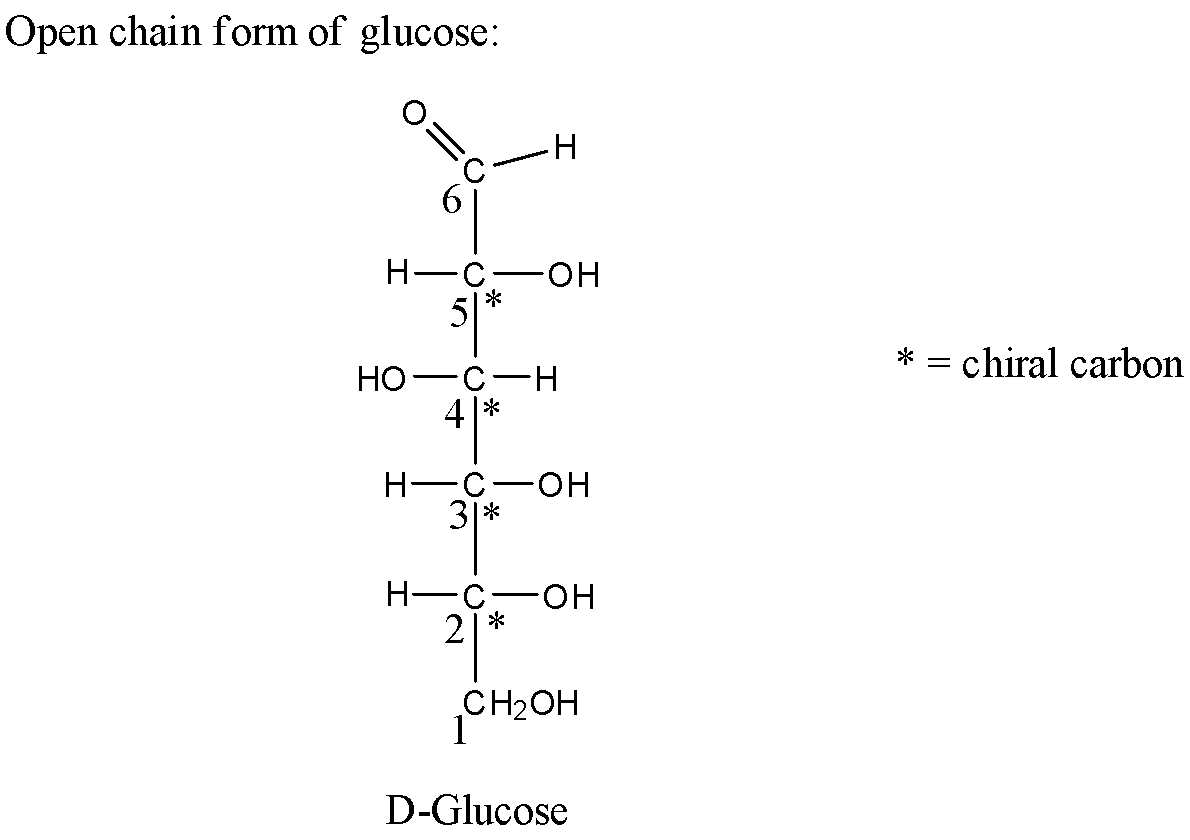
3) In the above structure the carbon has been labeled with numbers from below carbon. There is an aldehyde group attached to the ${6^{th}}$ carbon atom. Now let us analyze each carbon atom in the above structure and see for the chiral carbon.
4) Carbon ${1^{st}}$ is attached to two same hydrogen atoms hence it is not a chiral atom. Carbon ${2^{nd}}$ is attached to four different groups hence it is a chiral carbon atom. Similarly, carbon ${3^{rd}},{4^{th}},{5^{th}}$ is attached to four different groups hence they are chiral carbon atoms. In the case of carbon, ${6^{th}}$ there is a double-bonded oxygen atom attached which means that carbon is not a chiral carbon atom.
Therefore, there in the open-chain form of glucose there are four chiral carbon atoms present.
Note:
In the above structure the symmetry doesn’t pass through the molecule and one should always check this factor while deciding the chiral carbon atom. If the symmetry of the molecule passes through a carbon atom then it is not a chiral carbon atom.
Complete step by step answer:
1) First of all, we will try to understand the meaning of the chiral carbon atom. A chiral carbon atom must be attached to four different atoms or groups. If there are two or more than two same atoms or groups attached then it will not be a chiral carbon.
2) Now let us first see the structure of the open chain form of glucose as below,

3) In the above structure the carbon has been labeled with numbers from below carbon. There is an aldehyde group attached to the ${6^{th}}$ carbon atom. Now let us analyze each carbon atom in the above structure and see for the chiral carbon.
4) Carbon ${1^{st}}$ is attached to two same hydrogen atoms hence it is not a chiral atom. Carbon ${2^{nd}}$ is attached to four different groups hence it is a chiral carbon atom. Similarly, carbon ${3^{rd}},{4^{th}},{5^{th}}$ is attached to four different groups hence they are chiral carbon atoms. In the case of carbon, ${6^{th}}$ there is a double-bonded oxygen atom attached which means that carbon is not a chiral carbon atom.
Therefore, there in the open-chain form of glucose there are four chiral carbon atoms present.
Note:
In the above structure the symmetry doesn’t pass through the molecule and one should always check this factor while deciding the chiral carbon atom. If the symmetry of the molecule passes through a carbon atom then it is not a chiral carbon atom.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




