
What is the chemical formula of water?
A.${{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}$
B.${{{H}}_{{2}}}{{O}}$
C.${{{H}}_{{2}}}{{S}}$
D.${{{H}}_{{2}}}$
Answer
565.2k+ views
Hint: A chemical formula is the representation of elements that forms a compound. It represents the chemical proportion of the atoms present (the number of atoms of each element), how the elements are combined. By knowing the chemical formula, it is easy to name the compound. Similarly one can find the chemical formula of a compound from its molecular formula.
Complete step by step answer:
-We know that water molecules are made up of 2 elements, namely Hydrogen and Oxygen.
-When water is broken down through electrolysis, we get Hydrogen (${{{H}}_{{2}}}$) and oxygen (${{{O}}_2}$).
-The water molecule consists of 2 hydrogen atoms and 1 oxygen atom.
-We can draw the lewis dot structure of water.
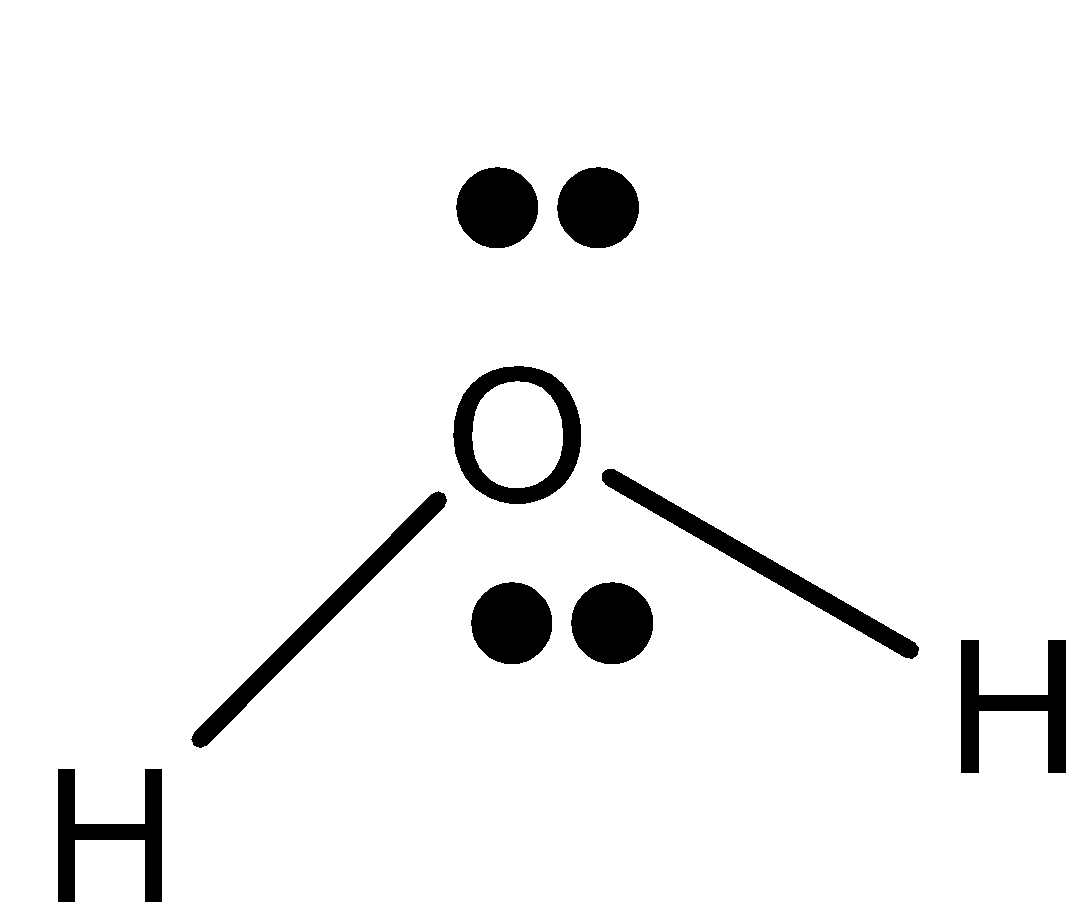
-We know that oxygen has 6 valence electrons in it. (${{1}}{{{s}}^2}2{{{s}}^2}2{{{p}}^4}$) , and the central atom is oxygen and hydrogen has 1 electron
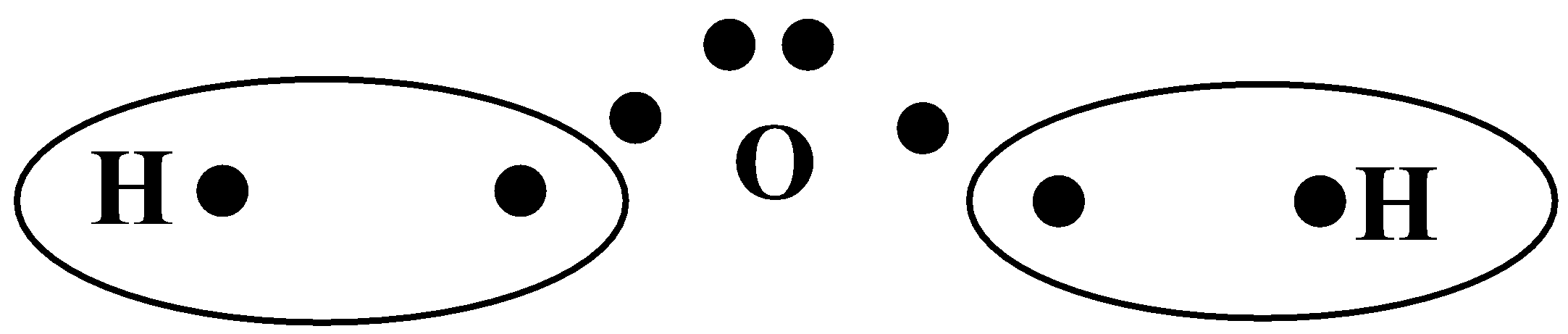
-Thus, water has 2 hydrogen atoms linked to an oxygen atom by a covalent bond (formed by the sharing of electrons).
-Since oxygen is more electronegative than hydrogen, all the electron density lies in the Oxygen atom. Oxygen has a partial positive charge and hydrogen has a partial negative charge.
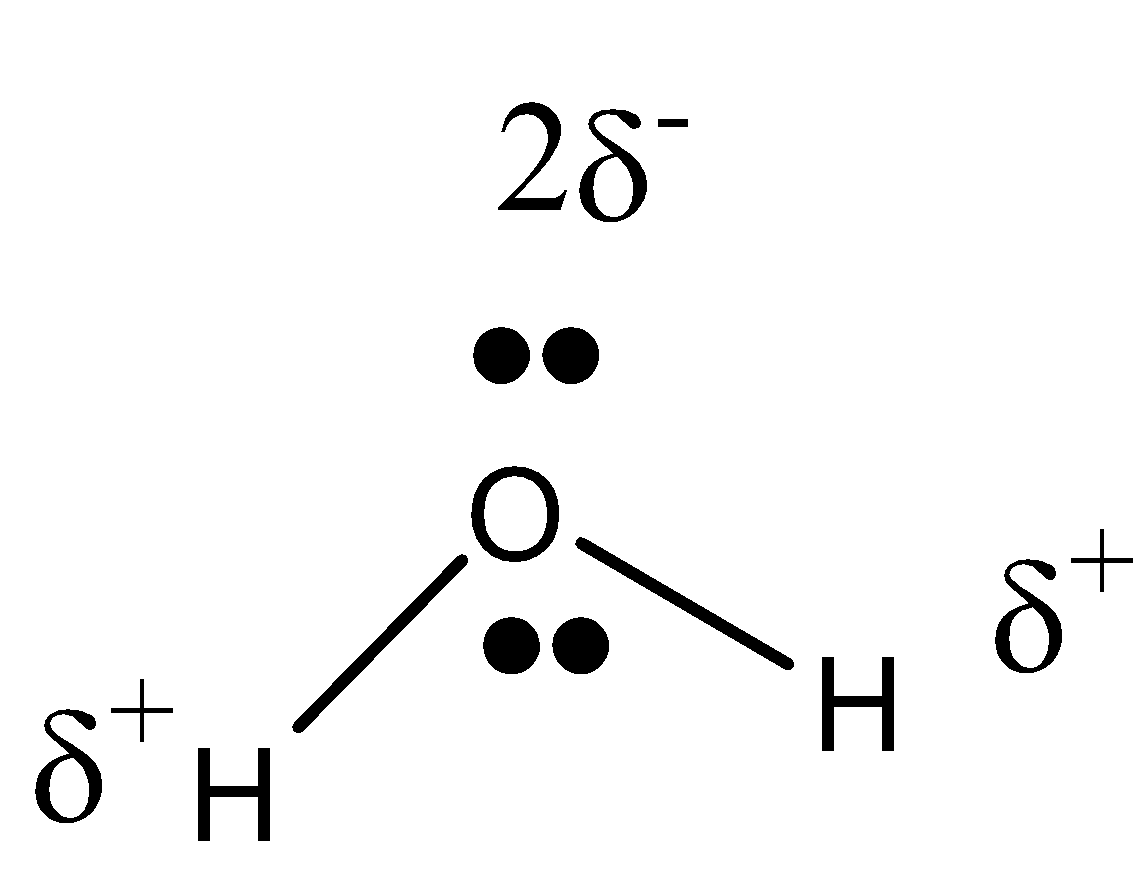
We already know that opposite charges attract each other. Since the water molecule has a charge, it attracts another molecule of water and is linked together by hydrogen bonds.
-${{2}}{{{H}}_{{2}}}{{ + }}{{{O}}_{{2}}}{{ }} \to {{ 2}}{{{H}}_{{2}}}{{O}}$
-Therefore, we can say that the chemical formula for water is ${{{H}}_2}{{O}}$.
The correct option is (B).
Note:
When hydrogen has 1 proton and 2 neutrons in its nucleus, (deuterium) forming a deuterium oxide with oxygen and is called heavy water (${{{D}}_{{2}}}{{O}}$ ). We know that there is hydrogen bonding, and water shows many properties. Water is a liquid at room temperature. Water is polar due to the presence of electronegative oxygen. This is the reason why some substances dissolve in water.
Complete step by step answer:
-We know that water molecules are made up of 2 elements, namely Hydrogen and Oxygen.
-When water is broken down through electrolysis, we get Hydrogen (${{{H}}_{{2}}}$) and oxygen (${{{O}}_2}$).
-The water molecule consists of 2 hydrogen atoms and 1 oxygen atom.
-We can draw the lewis dot structure of water.

-We know that oxygen has 6 valence electrons in it. (${{1}}{{{s}}^2}2{{{s}}^2}2{{{p}}^4}$) , and the central atom is oxygen and hydrogen has 1 electron

-Thus, water has 2 hydrogen atoms linked to an oxygen atom by a covalent bond (formed by the sharing of electrons).
-Since oxygen is more electronegative than hydrogen, all the electron density lies in the Oxygen atom. Oxygen has a partial positive charge and hydrogen has a partial negative charge.

We already know that opposite charges attract each other. Since the water molecule has a charge, it attracts another molecule of water and is linked together by hydrogen bonds.
-${{2}}{{{H}}_{{2}}}{{ + }}{{{O}}_{{2}}}{{ }} \to {{ 2}}{{{H}}_{{2}}}{{O}}$
-Therefore, we can say that the chemical formula for water is ${{{H}}_2}{{O}}$.
The correct option is (B).
Note:
When hydrogen has 1 proton and 2 neutrons in its nucleus, (deuterium) forming a deuterium oxide with oxygen and is called heavy water (${{{D}}_{{2}}}{{O}}$ ). We know that there is hydrogen bonding, and water shows many properties. Water is a liquid at room temperature. Water is polar due to the presence of electronegative oxygen. This is the reason why some substances dissolve in water.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




