
What is the chemical equation for the combustion of ${{C}_{3}}{{H}_{6}}$?
Answer
524.7k+ views
Hint: To solve this question we first need to know what is combustion. When a substance is reacted with oxygen gas, it releases energy in the form of heat and light. This reaction is called a combustion reaction.
Complete answer:
We know that ${{C}_{3}}{{H}_{6}}$ is the molecular formula for propene.
Propene is also called methyl ethylene or propylene.
It is unsaturated as it has 1 double bond and is the second simplest alkene after ethylene.
It has a petroleum-like faint odor and is a colorless gas.
The skeletal structure of propene is as follows
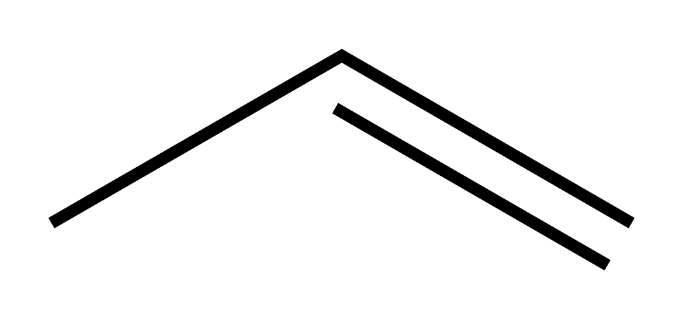
Propylene undergoes combustion reaction in a way similar to other alkenes.
- When there is sufficient oxygen, carbon dioxide and water is produced. The balanced chemical equation as follows
\[2{{C}_{3}}{{H}_{6}}+9{{O}_{2}}\to 6C{{O}_{2}}+6{{H}_{2}}O\]
- When there is limited oxygen such that complete combustion cannot take place, water, carbon monoxide and soot in the form of carbon is produced. The balanced chemical equation is as follows
\[{{C}_{3}}{{H}_{6}}+2{{O}_{2}}\to 3{{H}_{2}}O+2C+CO\]
Note:
It should be noted that apart from propene, ${{C}_{3}}{{H}_{6}}$ also exists as cyclopropane.
In cyclopropane, there are 3 methylene $C{{H}_{2}}$ groups attached to each other forming a ring. There exists a substantial ring strain due to the small size of the ring.
The skeletal structure of cyclopropane is as follows
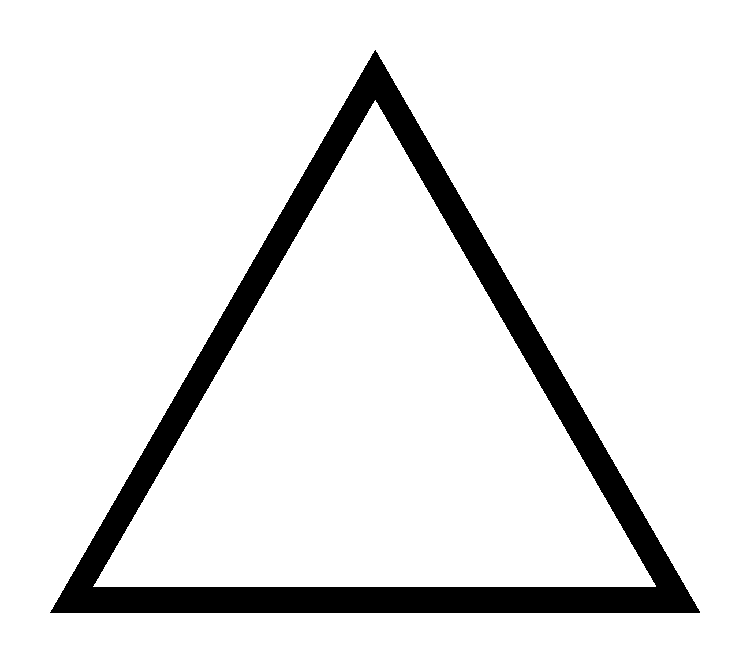
The products of combustion of cyclopropane are the same as the products formed during the combustion of propene i.e., water and carbon dioxide. The balanced chemical equation is as follows
\[2{{C}_{3}}{{H}_{6}}+9{{O}_{2}}\to 6C{{O}_{2}}+6{{H}_{2}}O\]
Complete answer:
We know that ${{C}_{3}}{{H}_{6}}$ is the molecular formula for propene.
Propene is also called methyl ethylene or propylene.
It is unsaturated as it has 1 double bond and is the second simplest alkene after ethylene.
It has a petroleum-like faint odor and is a colorless gas.
The skeletal structure of propene is as follows

Propylene undergoes combustion reaction in a way similar to other alkenes.
- When there is sufficient oxygen, carbon dioxide and water is produced. The balanced chemical equation as follows
\[2{{C}_{3}}{{H}_{6}}+9{{O}_{2}}\to 6C{{O}_{2}}+6{{H}_{2}}O\]
- When there is limited oxygen such that complete combustion cannot take place, water, carbon monoxide and soot in the form of carbon is produced. The balanced chemical equation is as follows
\[{{C}_{3}}{{H}_{6}}+2{{O}_{2}}\to 3{{H}_{2}}O+2C+CO\]
Note:
It should be noted that apart from propene, ${{C}_{3}}{{H}_{6}}$ also exists as cyclopropane.
In cyclopropane, there are 3 methylene $C{{H}_{2}}$ groups attached to each other forming a ring. There exists a substantial ring strain due to the small size of the ring.
The skeletal structure of cyclopropane is as follows

The products of combustion of cyclopropane are the same as the products formed during the combustion of propene i.e., water and carbon dioxide. The balanced chemical equation is as follows
\[2{{C}_{3}}{{H}_{6}}+9{{O}_{2}}\to 6C{{O}_{2}}+6{{H}_{2}}O\]
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




