
What is the charge, and Lewis structure of the nitrate anion?
Answer
503.7k+ views
Hint: The chemical formula for nitrate anion is \[N{O_3}^ - \]. The lewis dot structure marks the valence electrons of every element in the compound and the electrons which are involved in covalent and ionic bonds. We can use different symbols to show electrons of different elements.
Complete answer:
The nitrate ion is a polyatomic ion and the salts which contain this nitrate ion are called nitrates. The nitrate ion is a conjugate base of nitric acid. The chemical formula for nitrate anion is \[N{O_3}^ - \].
Lewis dot structure shows the bonding between atoms and molecules. It also represents the lone pair of electrons from the valence orbit.
In this question, we have to draw the lewis structure of nitrate anion \[N{O_3}^ - \]. The nitrate ion consists of one central atom i.e. nitrogen atom and it is surrounded by three identical bonded oxygen atoms. Its arrangement is trigonal planar. The nitrate ion has a formal charge of \[ - 1\]. The nitrate anion also shows resonance. It has three resonance structures.
We know that nitrogen has five valence electrons and oxygen has six valence electrons. Also, include the negative charge.
So, the total number of electrons = \[5 + 6 + 6 + 6 + 1 = 24\]
Structure of nitrate ion:
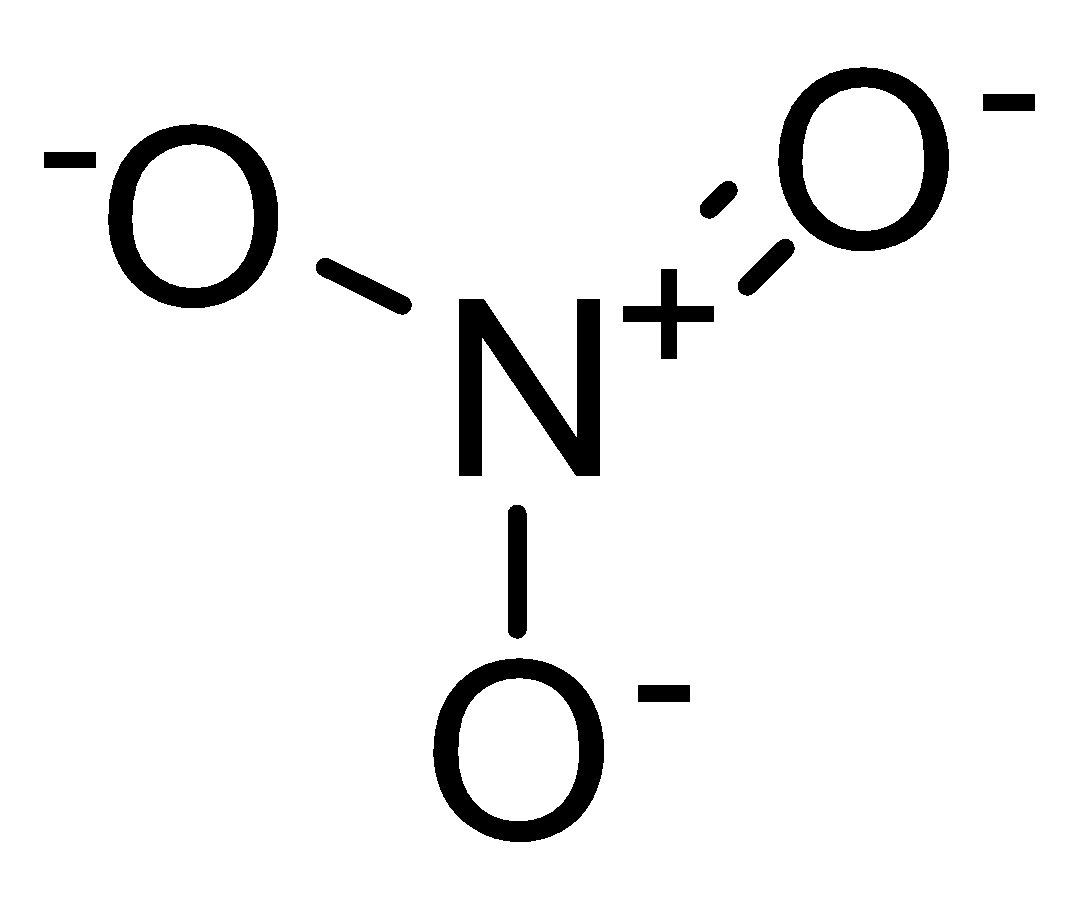
The double bonded oxygen has two lone pairs of electrons whereas a single bonded oxygen atom has three lone pairs of electrons. And the other electron forms a bond between nitrogen and oxygen.
Note:
The nitrate anion always has a negative charge due to its high electronegativity. Do not forget to include positive and negative while counting the total number of electrons. There are many applications of nitrate ions due to their high solubility and biodegradability. It is mainly used as fertilizers in agriculture. It is also used as oxidizing agents.
Complete answer:
The nitrate ion is a polyatomic ion and the salts which contain this nitrate ion are called nitrates. The nitrate ion is a conjugate base of nitric acid. The chemical formula for nitrate anion is \[N{O_3}^ - \].
Lewis dot structure shows the bonding between atoms and molecules. It also represents the lone pair of electrons from the valence orbit.
In this question, we have to draw the lewis structure of nitrate anion \[N{O_3}^ - \]. The nitrate ion consists of one central atom i.e. nitrogen atom and it is surrounded by three identical bonded oxygen atoms. Its arrangement is trigonal planar. The nitrate ion has a formal charge of \[ - 1\]. The nitrate anion also shows resonance. It has three resonance structures.
We know that nitrogen has five valence electrons and oxygen has six valence electrons. Also, include the negative charge.
So, the total number of electrons = \[5 + 6 + 6 + 6 + 1 = 24\]
Structure of nitrate ion:

The double bonded oxygen has two lone pairs of electrons whereas a single bonded oxygen atom has three lone pairs of electrons. And the other electron forms a bond between nitrogen and oxygen.
Note:
The nitrate anion always has a negative charge due to its high electronegativity. Do not forget to include positive and negative while counting the total number of electrons. There are many applications of nitrate ions due to their high solubility and biodegradability. It is mainly used as fertilizers in agriculture. It is also used as oxidizing agents.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




