
What is Catenation? Write the structural formulae of the following:
a) Cyclopropane
b) Ethane
c) Isobutane
Answer
597k+ views
Hint: Carbon shows the property of catenation. It can form long chains due to its valency 4. Due to this property vast no. of organic compounds are formed.
Complete step by step answer:
Binding of an element itself through covalent bonds in order to form chain and ring molecules are called Catenation. Carbon exhibits catenation property. It forms long hydrocarbon chains and rings like benzene. The bonds formed are covalent bonds. The ability of an element to possess the property of catenation is based on the bond energy of the element, which decreases with more diffused orbitals overlapping to form the bond. Carbon has least diffused valence shell p orbital which is capable of forming longer p-p sigma bonded chains of atoms than in heavier atoms. Catenation property is also influenced by a range of steric and electronic factors, including electronegativity of the element etc.
Sulphur can catenate to form monoclinic sulphur or \[{{S}_{8}}\] molecule. On heating these rings will open and can give rise to even longer chains.
Selenium and Tellurium also show catenation to a small extent.
Silicon can also form sigma bonds with other silicon atoms and form long chains. Its analogues to hydrocarbons are called silanes.
Structural formulae of
(A)Cyclopropane is \[{{C}_{3}}{{H}_{6}}\]
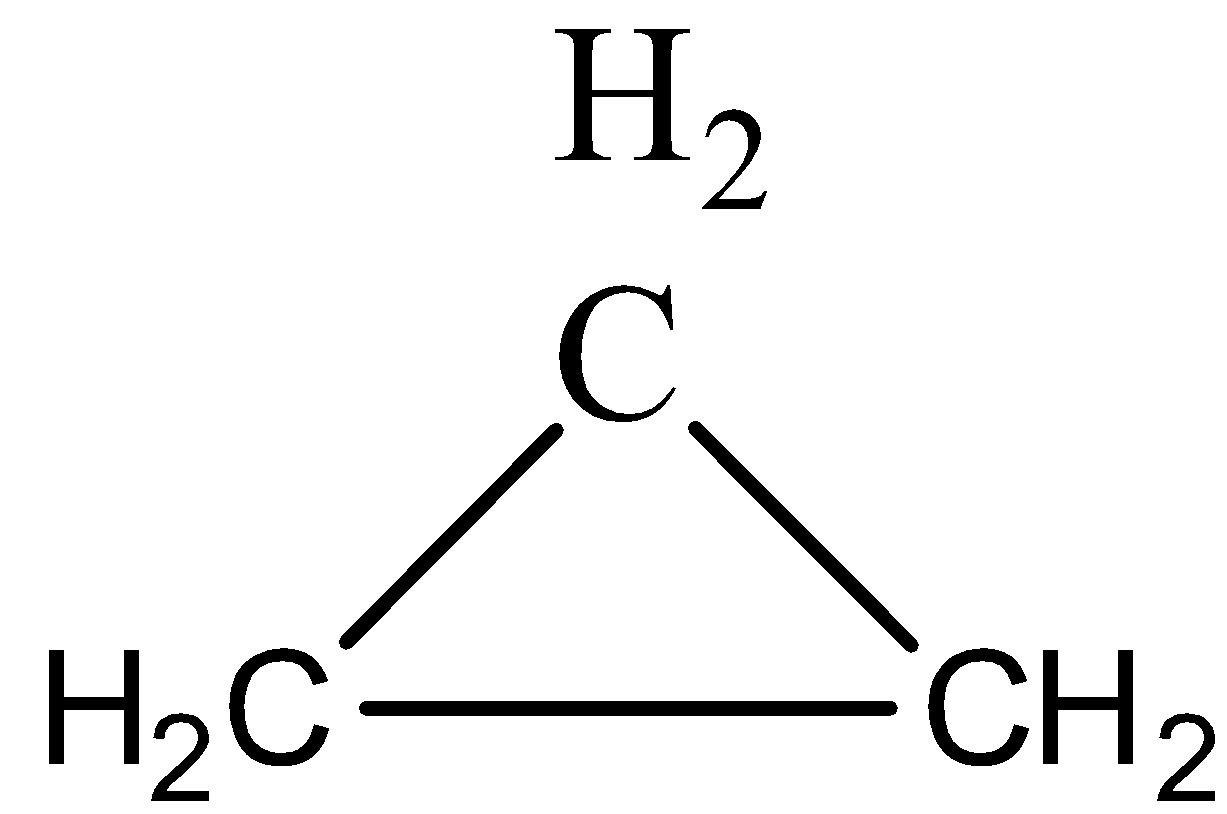
(B)Ethane is \[{{C}_{2}}{{H}_{6}}\]
\[{{H}_{3}}C-C{{H}_{3}}\]
(C)Isobutane is \[{{C}_{4}}{{H}_{10}}\]
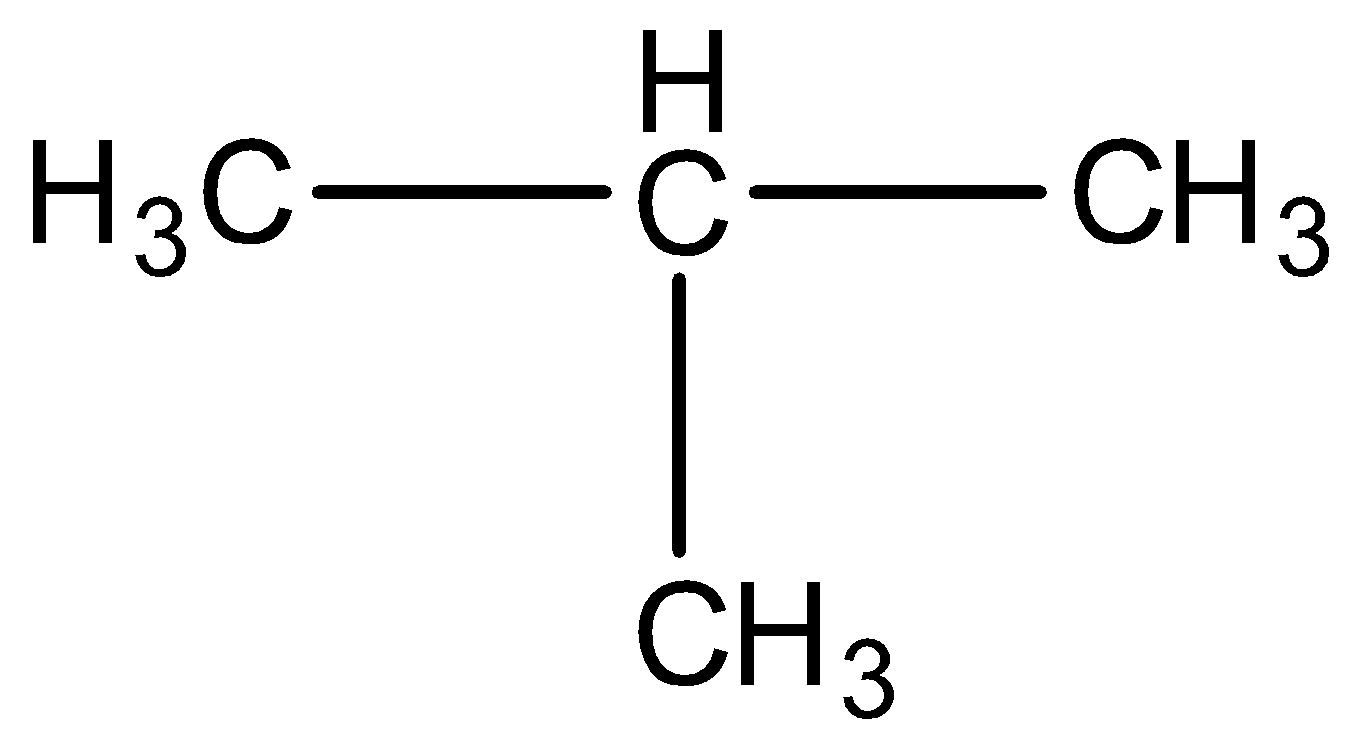
Structural formula is straight lines connecting the atomic symbols which are used to represent single bond, two such lines to represent double bond and 3 lines for triple bond. Such representations of a compound is called structural formula of a compound
Note: Structural formula is a way of representing chemical compounds. While writing the structural formula, we need to check the number atoms that are present and how they are bonded in the molecule. It is derived from the IUPAC name.
Complete step by step answer:
Binding of an element itself through covalent bonds in order to form chain and ring molecules are called Catenation. Carbon exhibits catenation property. It forms long hydrocarbon chains and rings like benzene. The bonds formed are covalent bonds. The ability of an element to possess the property of catenation is based on the bond energy of the element, which decreases with more diffused orbitals overlapping to form the bond. Carbon has least diffused valence shell p orbital which is capable of forming longer p-p sigma bonded chains of atoms than in heavier atoms. Catenation property is also influenced by a range of steric and electronic factors, including electronegativity of the element etc.
Sulphur can catenate to form monoclinic sulphur or \[{{S}_{8}}\] molecule. On heating these rings will open and can give rise to even longer chains.
Selenium and Tellurium also show catenation to a small extent.
Silicon can also form sigma bonds with other silicon atoms and form long chains. Its analogues to hydrocarbons are called silanes.
Structural formulae of
(A)Cyclopropane is \[{{C}_{3}}{{H}_{6}}\]

(B)Ethane is \[{{C}_{2}}{{H}_{6}}\]
\[{{H}_{3}}C-C{{H}_{3}}\]
(C)Isobutane is \[{{C}_{4}}{{H}_{10}}\]

Structural formula is straight lines connecting the atomic symbols which are used to represent single bond, two such lines to represent double bond and 3 lines for triple bond. Such representations of a compound is called structural formula of a compound
Note: Structural formula is a way of representing chemical compounds. While writing the structural formula, we need to check the number atoms that are present and how they are bonded in the molecule. It is derived from the IUPAC name.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




