
How would you carry out the following conversion?
Benzyl chloride to benzyl alcohol
Answer
522.9k+ views
Hint: Conversion of aryl halide to aryl alcohol is an example for substitution chemical reaction. To substitute the halide from the aryl halide there is a need for a strong base. By using this reaction we can prepare alcohol from halides.
Complete answer:
- In the question it is asked to do the conversion of benzyl chloride to benzyl alcohol.
- First we should know the structure of the benzyl chloride.
- The structure of the benzyl chloride is as follows.
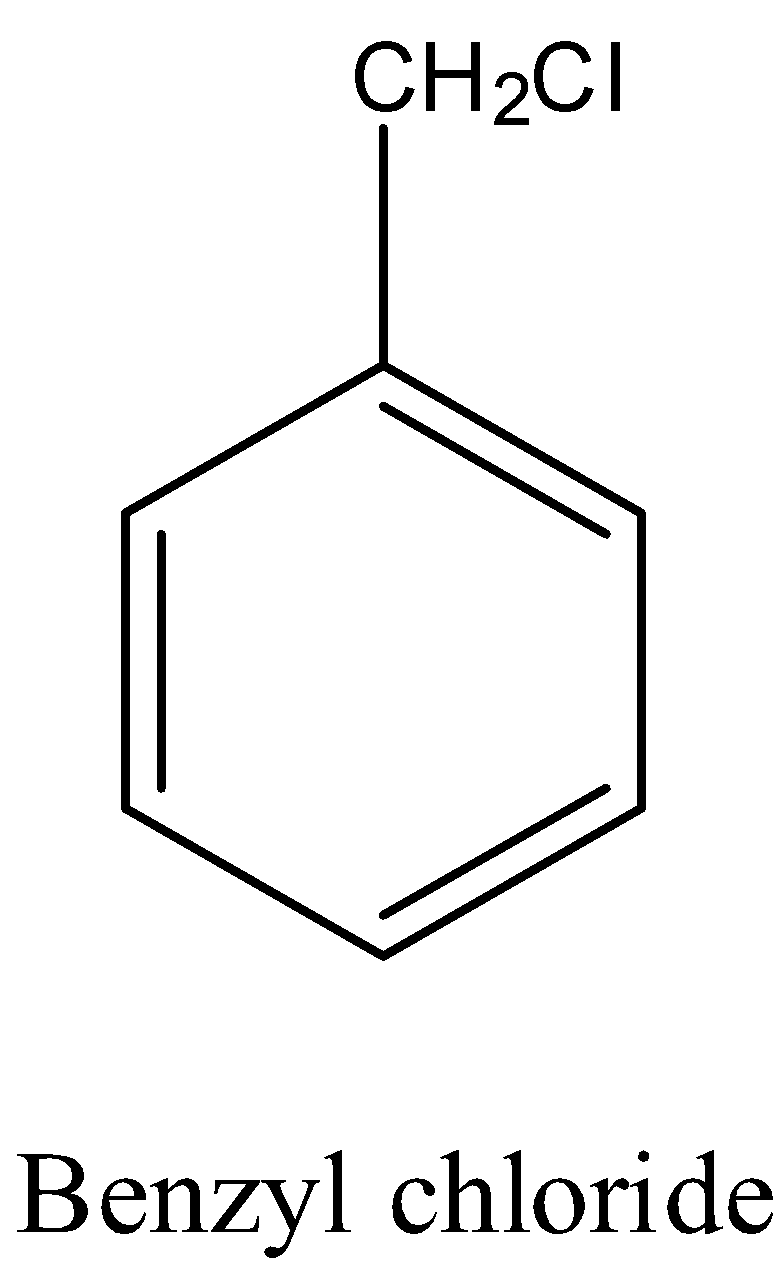
- Now we can see the preparation of the benzyl alcohol from the benzyl chloride with the help of aqueous potassium hydroxide and it is as follows.
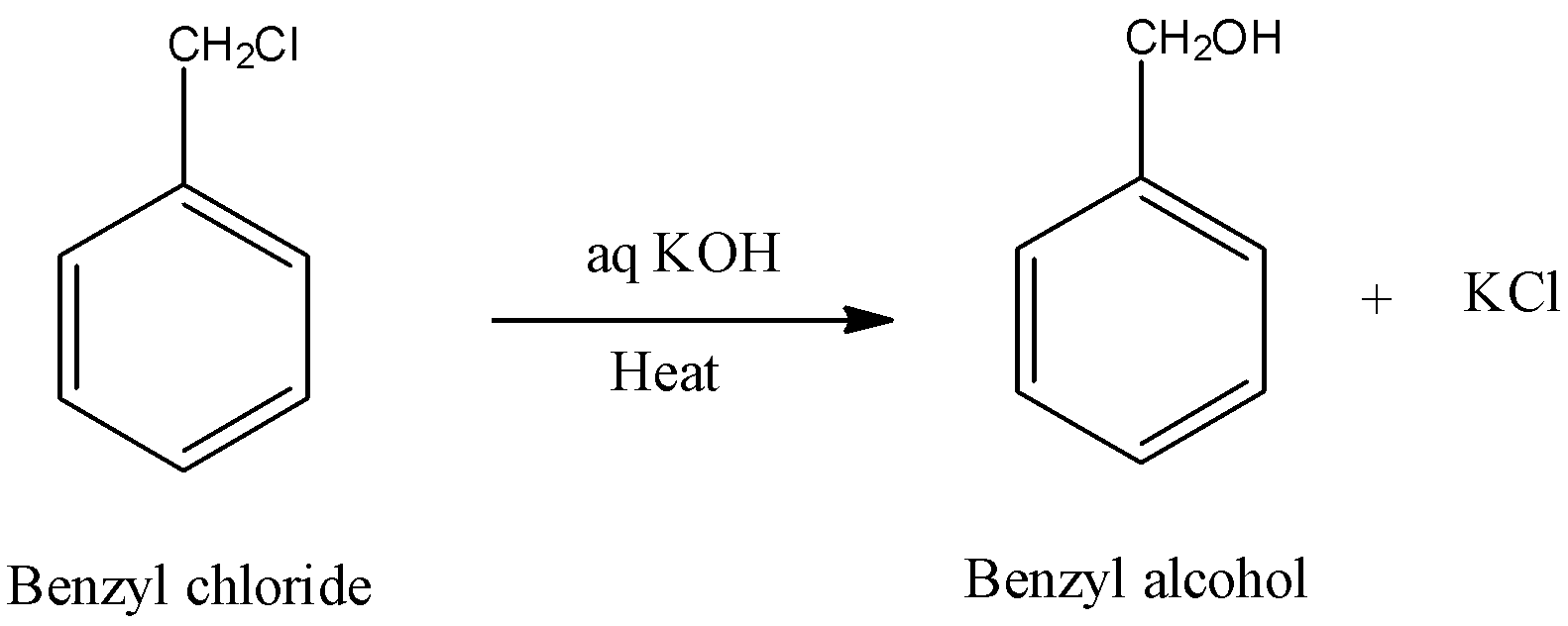
- In the above chemical reaction we can see that one mole of benzoyl chloride is going to convert into one mole of benzyl alcohol with the help of the aqueous potassium hydroxide.
- At the same time there is a formation of the byproduct potassium chloride (KCl).
- The above mentioned chemical reaction is going to be carried out with the help of some energy which is going to be supplied in the form of a heat.
- Therefore the conversion of benzyl chloride to benzyl alcohol is an example for endothermic chemical reaction.
Note:
If the energy is going to be liberated in the form of a heat during the chemical reaction then the chemical reaction is called exothermic reaction and in the conversion of benzoyl chloride to benzyl alcohol the energy is going to be absorbed.
Complete answer:
- In the question it is asked to do the conversion of benzyl chloride to benzyl alcohol.
- First we should know the structure of the benzyl chloride.
- The structure of the benzyl chloride is as follows.

- Now we can see the preparation of the benzyl alcohol from the benzyl chloride with the help of aqueous potassium hydroxide and it is as follows.

- In the above chemical reaction we can see that one mole of benzoyl chloride is going to convert into one mole of benzyl alcohol with the help of the aqueous potassium hydroxide.
- At the same time there is a formation of the byproduct potassium chloride (KCl).
- The above mentioned chemical reaction is going to be carried out with the help of some energy which is going to be supplied in the form of a heat.
- Therefore the conversion of benzyl chloride to benzyl alcohol is an example for endothermic chemical reaction.
Note:
If the energy is going to be liberated in the form of a heat during the chemical reaction then the chemical reaction is called exothermic reaction and in the conversion of benzoyl chloride to benzyl alcohol the energy is going to be absorbed.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




