
Carbon tetrachloride ($CC{{l}_{4}}$ ):
(A) ionic substance
(B) non-polar covalent substance
(C) polar covalent substance
(D) macromolecular substance
(E) metallic substance
Answer
596.7k+ views
Hint: A dipole moment occurs when there is a separation of charge. They can occur between two ions in an ionic bond or between two atoms in a covalent bond; dipole moment rises from a difference in electronegativity. Larger dipole moment with the larger difference in electronegativity of atoms involved in bond formation.
Complete step by step solution:
When a covalent bond is formed between two similar atoms ${{H}_{2}}, C{{l}_{2}}\And {{O}_{2}}$ the shared pair electrons equally attracted by two atoms. The bond is called a nonpolar covalent bond.
As a result of polarization, the molecule possesses the dipole moment, a measure of the polarity of the molecule. The distance between the charge separation is also a deciding factor in the size of the dipole moment.
Dipole Moment ( $\mu $ ) = charge (Q) X distance separation (r)
The dipole moment is usually expressed in the Debye unit (D).
The conversion factor, 1D = $3.33564X{{10}^{-30}}Cm$ , where C is coulomb and m is a meter
When a molecule consists of more than one polar pond, maybe the polarity of the bond cancels with another. While in the case of polyatomic molecules like carbon tetrachloride, polarity will decide based on the structure of the molecule. In such a case, it is necessary to treat each bond as a vector and it indicates with an arrow. The arrow shows the length of the molecule and the direction shows from positive to negative end, such that adding an individual bond dipole moment as a vector will give the overall dipole moment of the molecule.
The hybridization of carbon tetrachloride is $s{{p}^{3}}$, its structure is tetrahedral.
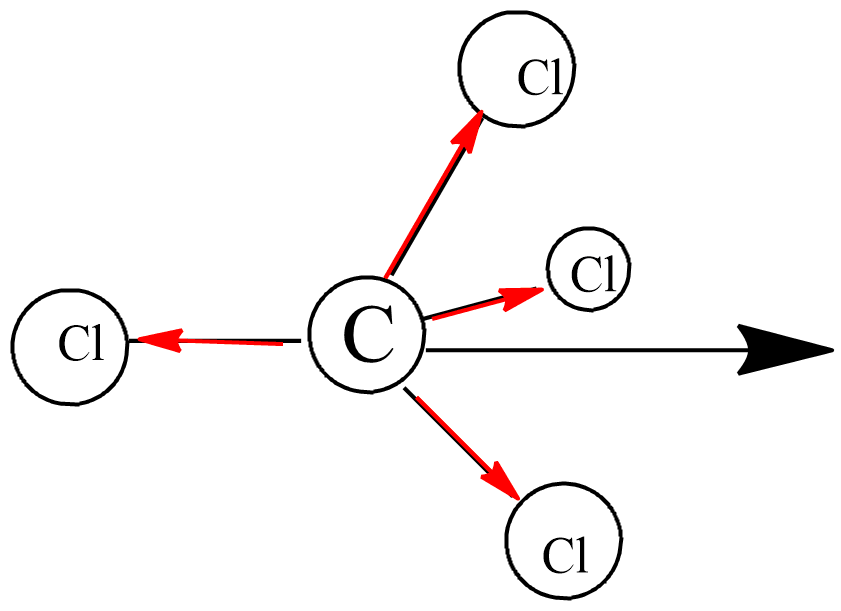
The tetrahedral structure of carbon tetrachloride $CC{{l}_{4}}$ shows in the figure above. This figure indicates the simplest arrangements of equivalent bonds around the central atom with a resulting dipole moment of zero. Because in the tetrahedral the polarity of three right-hand bonds cancels the left-handed bond polarity. So, carbon tetrachloride is a nonpolar covalent bond.
The correct answer is option B.
Note: A nonpolar covalent bond is a type of covalent bond because the separation of charges between two or more atoms due to the dipole moment is zero. The type of covalent bond when polar bonds arrange themselves such that cancel each other.
Complete step by step solution:
When a covalent bond is formed between two similar atoms ${{H}_{2}}, C{{l}_{2}}\And {{O}_{2}}$ the shared pair electrons equally attracted by two atoms. The bond is called a nonpolar covalent bond.
As a result of polarization, the molecule possesses the dipole moment, a measure of the polarity of the molecule. The distance between the charge separation is also a deciding factor in the size of the dipole moment.
Dipole Moment ( $\mu $ ) = charge (Q) X distance separation (r)
The dipole moment is usually expressed in the Debye unit (D).
The conversion factor, 1D = $3.33564X{{10}^{-30}}Cm$ , where C is coulomb and m is a meter
When a molecule consists of more than one polar pond, maybe the polarity of the bond cancels with another. While in the case of polyatomic molecules like carbon tetrachloride, polarity will decide based on the structure of the molecule. In such a case, it is necessary to treat each bond as a vector and it indicates with an arrow. The arrow shows the length of the molecule and the direction shows from positive to negative end, such that adding an individual bond dipole moment as a vector will give the overall dipole moment of the molecule.
The hybridization of carbon tetrachloride is $s{{p}^{3}}$, its structure is tetrahedral.

The tetrahedral structure of carbon tetrachloride $CC{{l}_{4}}$ shows in the figure above. This figure indicates the simplest arrangements of equivalent bonds around the central atom with a resulting dipole moment of zero. Because in the tetrahedral the polarity of three right-hand bonds cancels the left-handed bond polarity. So, carbon tetrachloride is a nonpolar covalent bond.
The correct answer is option B.
Note: A nonpolar covalent bond is a type of covalent bond because the separation of charges between two or more atoms due to the dipole moment is zero. The type of covalent bond when polar bonds arrange themselves such that cancel each other.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




