
Carbon suboxide \[{C_3}{O_2}\] has:
A.Linear structure
B.Bent structure
C.Trigonal planar structure
D.Distorted tetrahedral structure
Answer
591.6k+ views
Hint: Carbon suboxide is also known as tri carbon dioxide. It is a type of linear oxo carbon, which is represented by the chemical formula \[O = {C_n} = O\] . In the case of carbon suboxide, the value of n is equal to 3. Carbon dioxide also belongs to this group of carbon compounds known as oxo carbon, with the value of n equating to 1.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
The structure of carbon can be explained as a chain of carbon atoms that are linked with each other using one sigma bond and one pi bond, with doubly bonded oxygen atoms at the terminal positions of the chain.
Thus, a simpler rendition of the molecule of \[{C_3}{O_2}\] or carbon suboxide is \[O = C = C = C = O\] .
Since the molecule of carbon suboxide is formed from a single chain, it is expected to have basic two – dimensional geometries. Hence, the possibilities for this molecule to exhibit three – dimensional geometries like trigonal planar and distorted tetrahedral can be completely ruled out. Now to determine the exact geometry of this molecule, let us understand the stability of this molecule. This molecule has one lone pair of electrons on each of the oxygen atoms, and pi bonds adjacent to these lone pairs. This results in the formation of resonance structures. These resonance structures can be represented as:
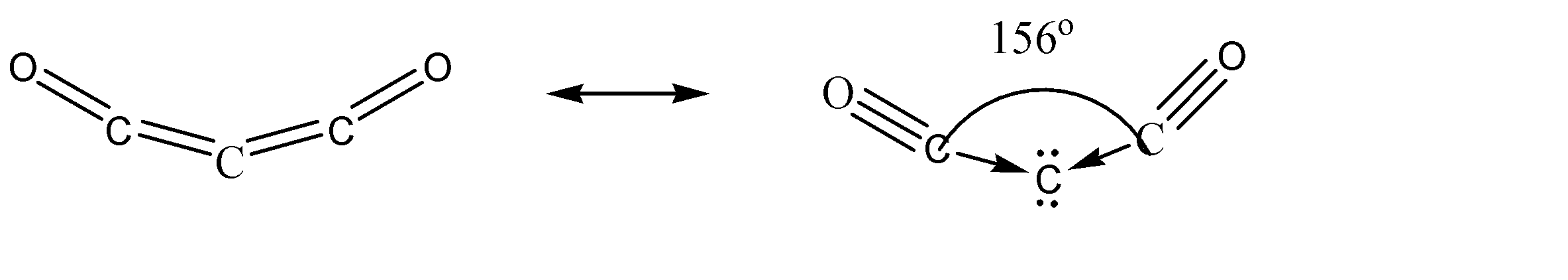
The resonance structures cause a certain amount of bending characteristic in the structure due to the variation in the strengths of the triple bonds and the coordinate bonds.
Hence, the molecule of carbon suboxide has a bent structure.
Hence, Option D is the correct option
Note: Carbon suboxide is synthesized by warming a dry mixture of phosphorus pentoxide and malonic acid or its esters. Therefore, it can be also considered as the anhydride of malonic anhydride, or in simpler terms, the second anhydride of malonic acid.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
The structure of carbon can be explained as a chain of carbon atoms that are linked with each other using one sigma bond and one pi bond, with doubly bonded oxygen atoms at the terminal positions of the chain.
Thus, a simpler rendition of the molecule of \[{C_3}{O_2}\] or carbon suboxide is \[O = C = C = C = O\] .
Since the molecule of carbon suboxide is formed from a single chain, it is expected to have basic two – dimensional geometries. Hence, the possibilities for this molecule to exhibit three – dimensional geometries like trigonal planar and distorted tetrahedral can be completely ruled out. Now to determine the exact geometry of this molecule, let us understand the stability of this molecule. This molecule has one lone pair of electrons on each of the oxygen atoms, and pi bonds adjacent to these lone pairs. This results in the formation of resonance structures. These resonance structures can be represented as:

The resonance structures cause a certain amount of bending characteristic in the structure due to the variation in the strengths of the triple bonds and the coordinate bonds.
Hence, the molecule of carbon suboxide has a bent structure.
Hence, Option D is the correct option
Note: Carbon suboxide is synthesized by warming a dry mixture of phosphorus pentoxide and malonic acid or its esters. Therefore, it can be also considered as the anhydride of malonic anhydride, or in simpler terms, the second anhydride of malonic acid.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




