
Carbon suboxide ${C_3}{O_2}$ has:
A: Linear structure
B: Bent structure
C: Trigonal planar structure
D: Distorted tetrahedral structure
Answer
585.9k+ views
Hint: In carbon suboxide three atoms of carbon are linked with two atoms of oxygen. In this compound there are double bonds between the atoms. All the structures given in options are formed when there is no lone pair on the central atom.
Complete step by step answer:
Carbon suboxide is a colorless gas. It has a strong pungent smell. This gas reacts with water to give malonic acid. Carbon suboxide is one of the stable members of the series of linear oxocarbons. Oxo Carbons are represented by the formula $O = {C_n} = O$. Chemical formula of carbon suboxide is ${C_3}{O_2}$. In this molecule there are three atoms of carbon and two atoms of oxygen. Structure of this compound is as follows:
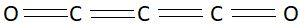
In this picture we can clearly see that carbon suboxide has linear structure.
So, the correct answer is Option A .
Additional Information:
Carbon suboxide is a foul smelling gas which is produced by the dehydration of malonic acid, $C{H_2}{\left( {COOH} \right)_2}$, with ${P_4}{O_{10}}$ in vacuum. At ${25^ \circ }C$ this compound polymerises to a highly color solid substance. Under the influence of ultraviolet light this compound decomposes to ketene. Ketene is a highly reactive compound. Carbon suboxide is an acid anhydride of malonic acid. Carbon suboxide reacts slowly with water to give malonic acid. Carbon suboxide has a bent structure in gaseous phase.
Note:
Carbon dioxide is also a molecule of carbon and oxygen. Chemical formula of this molecule is $C{O_2}$. Like carbon suboxide, carbon dioxide also has linear structure. Hybridization of this molecule is $sp$. In this molecule there is no lone pair on the central atom.
Complete step by step answer:
Carbon suboxide is a colorless gas. It has a strong pungent smell. This gas reacts with water to give malonic acid. Carbon suboxide is one of the stable members of the series of linear oxocarbons. Oxo Carbons are represented by the formula $O = {C_n} = O$. Chemical formula of carbon suboxide is ${C_3}{O_2}$. In this molecule there are three atoms of carbon and two atoms of oxygen. Structure of this compound is as follows:
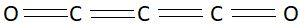
In this picture we can clearly see that carbon suboxide has linear structure.
So, the correct answer is Option A .
Additional Information:
Carbon suboxide is a foul smelling gas which is produced by the dehydration of malonic acid, $C{H_2}{\left( {COOH} \right)_2}$, with ${P_4}{O_{10}}$ in vacuum. At ${25^ \circ }C$ this compound polymerises to a highly color solid substance. Under the influence of ultraviolet light this compound decomposes to ketene. Ketene is a highly reactive compound. Carbon suboxide is an acid anhydride of malonic acid. Carbon suboxide reacts slowly with water to give malonic acid. Carbon suboxide has a bent structure in gaseous phase.
Note:
Carbon dioxide is also a molecule of carbon and oxygen. Chemical formula of this molecule is $C{O_2}$. Like carbon suboxide, carbon dioxide also has linear structure. Hybridization of this molecule is $sp$. In this molecule there is no lone pair on the central atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




