
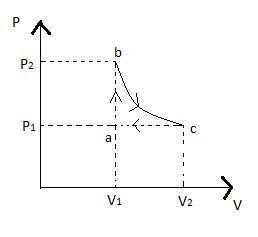
Carbon monoxide is carried around a closed abc in which bc is an isothermal process, as shown in fig. The gas absorbs 7000J of heat as its temperature increases from 300 K to 1000 K in going from a to b. The quantity of heat rejected by the gas during the process CA Is
A. 4200 J
B. 5000 J
C. 9000 J
D. 9800 J

Answer
583.5k+ views
Hint: In this question, the PV cycle is given where curve bc going through the isothermal process, ab is going through the isochoric process since volume is constant, and ca be going through the isobaric process as pressure is constant.
Complete step by step answer:
Given in cycle abc, bc is an isothermal process
In cycle, we can see ab is an isochoric process since volume is constant where 7000J of heat is absorbed as its temperature increases from 300 K to 1000 K
Hence isochoric process for AB will be
\[\Delta {U_{AB}} = \Delta {Q_{AB}} - \Delta {W_{AB}} - - (i)\]
Where
\[\Delta {W_{AB}} = 0\], since the area under the curve, a to b is zero
\[\Delta {Q_{AB}} = 7000J\]is given
Hence we can write equation (i) as
\[
\Delta {U_{AB}} = \Delta {Q_{AB}} - 0 \\
\Delta {U_{AB}} = \Delta {Q_{AB}} = 7000J - - (ii) \\
\]
Now for the isochoric process, we know
\[\Delta U = n{C_v}\Delta T - - (iii)\]
Hence we can write equation (iii) for the curve AB as
\[
\Delta {U_{AB}} = n{C_v}\Delta T \\
= n\dfrac{5}{2}R\left( {1000 - 300} \right) \\
\]
Where\[\Delta {U_{AB}} = 7000J\], from equation (ii), hence we get
\[
7000 = n\dfrac{5}{2}R\left( {1000 - 300} \right) \\
n\dfrac{5}{2}R700 = 7000 \\
nR = \dfrac{{20}}{5} \\
nR = 4 - - (iv) \\
\]
Now for curve ac, since the pressure is constant, so the process is isobaric, this is given as
\[\Delta {U_{AC}} = n{C_p}\Delta T - - (v)\]
Since the curve bc is isothermal hence we can say temperature at b will be equal to the temperature at c; hence we can write equation (v) as
\[
\Delta {U_{AC}} = n{C_p}\Delta T \\
= n\dfrac{7}{2}R\left( {300 - 1000} \right) \\
= - nR\dfrac{7}{2}700 \\
\]
Where the value of \[nR = 4\]from equation (iv), hence we can write
\[
\Delta {U_{AC}} = - nR\dfrac{7}{2}700 \\
= - 4 \times \dfrac{7}{2} \times 700 \\
= - 9800J \\
\]
So the quantity of heat rejected by the gas during the process ca is \[ = 9800J\]
Option D is correct.
Note:The process in which there is no change in the pressure or the process in which the pressure remains constant is known as Isobaric Process.
The process in which there is no change in the volume or the process in which the volume remains constant is known as Isochoric Process.
Complete step by step answer:
Given in cycle abc, bc is an isothermal process
In cycle, we can see ab is an isochoric process since volume is constant where 7000J of heat is absorbed as its temperature increases from 300 K to 1000 K
Hence isochoric process for AB will be
\[\Delta {U_{AB}} = \Delta {Q_{AB}} - \Delta {W_{AB}} - - (i)\]
Where
\[\Delta {W_{AB}} = 0\], since the area under the curve, a to b is zero
\[\Delta {Q_{AB}} = 7000J\]is given
Hence we can write equation (i) as
\[
\Delta {U_{AB}} = \Delta {Q_{AB}} - 0 \\
\Delta {U_{AB}} = \Delta {Q_{AB}} = 7000J - - (ii) \\
\]
Now for the isochoric process, we know
\[\Delta U = n{C_v}\Delta T - - (iii)\]
Hence we can write equation (iii) for the curve AB as
\[
\Delta {U_{AB}} = n{C_v}\Delta T \\
= n\dfrac{5}{2}R\left( {1000 - 300} \right) \\
\]
Where\[\Delta {U_{AB}} = 7000J\], from equation (ii), hence we get
\[
7000 = n\dfrac{5}{2}R\left( {1000 - 300} \right) \\
n\dfrac{5}{2}R700 = 7000 \\
nR = \dfrac{{20}}{5} \\
nR = 4 - - (iv) \\
\]
Now for curve ac, since the pressure is constant, so the process is isobaric, this is given as
\[\Delta {U_{AC}} = n{C_p}\Delta T - - (v)\]
Since the curve bc is isothermal hence we can say temperature at b will be equal to the temperature at c; hence we can write equation (v) as
\[
\Delta {U_{AC}} = n{C_p}\Delta T \\
= n\dfrac{7}{2}R\left( {300 - 1000} \right) \\
= - nR\dfrac{7}{2}700 \\
\]
Where the value of \[nR = 4\]from equation (iv), hence we can write
\[
\Delta {U_{AC}} = - nR\dfrac{7}{2}700 \\
= - 4 \times \dfrac{7}{2} \times 700 \\
= - 9800J \\
\]
So the quantity of heat rejected by the gas during the process ca is \[ = 9800J\]
Option D is correct.
Note:The process in which there is no change in the pressure or the process in which the pressure remains constant is known as Isobaric Process.
The process in which there is no change in the volume or the process in which the volume remains constant is known as Isochoric Process.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




