
Carbon has four electrons in its valence shell. Which type of compounds can be formed by carbon atoms and why? Give any one example of such compounds.
Answer
544.8k+ views
Hint:In the above question, it is given that carbon has four electrons in the valence shell. We have to find which type of compound is formed by carbon. Every element in the periodic table tries to achieve their complete octet state by gaining, losing or sharing electrons.
Complete step-by-step answer:We know that every element tries to achieve their octet state. And hence, the elements having more than 4 electrons in the valence shell gain electrons as gaining electrons is easier than losing them. In case, the elements have less than 4 electrons in the valence shell, it loses the electrons instead of gaining as the energy required in losing the electrons is less than gaining electrons.
But carbon has 4 electrons in the valence shell. It can neither lose electrons nor it can gain electrons and hence, it shares electrons. So, covalent compounds can be formed by carbon atoms.
Example: Methane.
Carbon has 4 valence electrons and hydrogen has 1 electron. To complete its octet, carbon requires 4 electrons and hydrogen requires only 1 electron. So, the central carbon atom bonds with 4 hydrogen atoms so that both compounds can achieve their complete octet state.
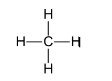
Note:Most of the things which surround us are basically covalent compounds. For example, plastic, petroleum and water. Even the food that we eat, the oxygen that we inhale, the carbon dioxide that we exhale are examples of covalently bonded molecules.
Complete step-by-step answer:We know that every element tries to achieve their octet state. And hence, the elements having more than 4 electrons in the valence shell gain electrons as gaining electrons is easier than losing them. In case, the elements have less than 4 electrons in the valence shell, it loses the electrons instead of gaining as the energy required in losing the electrons is less than gaining electrons.
But carbon has 4 electrons in the valence shell. It can neither lose electrons nor it can gain electrons and hence, it shares electrons. So, covalent compounds can be formed by carbon atoms.
Example: Methane.
Carbon has 4 valence electrons and hydrogen has 1 electron. To complete its octet, carbon requires 4 electrons and hydrogen requires only 1 electron. So, the central carbon atom bonds with 4 hydrogen atoms so that both compounds can achieve their complete octet state.

Note:Most of the things which surround us are basically covalent compounds. For example, plastic, petroleum and water. Even the food that we eat, the oxygen that we inhale, the carbon dioxide that we exhale are examples of covalently bonded molecules.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




