
How is carbolic acid prepared from the following compound?
A) Aniline
B) Chlorobenzene and steam at $698K$ ?
C) Draw structure of DDT. Write its environmental effects.
D) Mention two physical properties of carbolic acid.
Answer
564.9k+ views
Hint:In order to answer this question you must be aware of the preparation of Carboxylic Acids. Recall the method where you have prepared carboxylic acid from Aniline and also by chlorobenzene and steam at $698K$ . And then recall the structure and properties of DDT, draw its structure and write its impact on our environment. And at last Write any two properties of carbolic acid.
Complete solution:
Step 1: In this step we will write the preparation of carboxylic acid from Aniline:
Firstly, treat Aniline with $NaN{O_2}/HCl$ and make the diazonium ion, then treat the diazonium ion with $CuCN$ .
Secondly, Hydrolyze the $CN$ group to the carboxylic acid.
Finally, you will obtain the product as Benzoic Acid.
The reaction takes place according to the following mechanism:
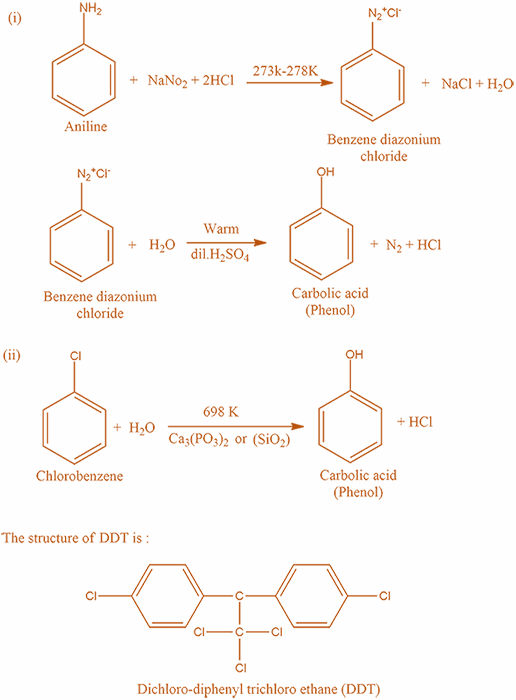
Step 2: In this step we will write the preparation of carboxylic acid from Chlorobenzene and steam at $698K$ :
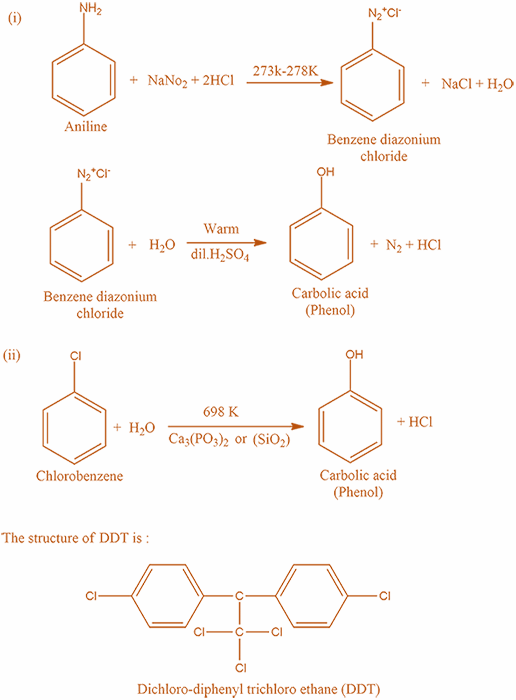
Firstly, treat Chlorobenzene is heated with excess of Aqueous Sodium hydroxide $NaOH$ at $698K$ then sodium phenoxide is formed.
Secondly, treat Sodium Phenoxide with dilute $HCl$ , and hence Phenol ( also known as carbolic acid ) is formed.
The reaction takes place according to the following mechanism:
Step 3: Structure of DDT:
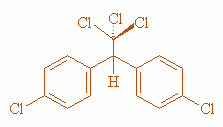
Effects of DDT on environment are:
1) It is non-biodegradable in nature.
2) It is deposited and stored in fatty tissues.
Step 4: Two physical properties of carbolic acid:
1) It is a colourless, hygroscopic, crystalline solid with typical phenolic odour. On exposure to air and light, it turns pink due to partial oxidation.
2) It has low melting point, but has higher boiling point due to intermolecular hydrogen bonding.
Note:Dichlorodiphenyltrichloroethane, commonly known as DDT, is a colorless, tasteless, and almost odorless crystalline chemical compound, an organochlorine. Originally developed as an insecticide, it became infamous for its environmental impacts. Phenol is an aromatic organic compound with the molecular formula ${C_6}{H_5}OH$ . It is a white crystalline solid that is volatile.
Complete solution:
Step 1: In this step we will write the preparation of carboxylic acid from Aniline:
Firstly, treat Aniline with $NaN{O_2}/HCl$ and make the diazonium ion, then treat the diazonium ion with $CuCN$ .
Secondly, Hydrolyze the $CN$ group to the carboxylic acid.
Finally, you will obtain the product as Benzoic Acid.
The reaction takes place according to the following mechanism:

Step 2: In this step we will write the preparation of carboxylic acid from Chlorobenzene and steam at $698K$ :

Firstly, treat Chlorobenzene is heated with excess of Aqueous Sodium hydroxide $NaOH$ at $698K$ then sodium phenoxide is formed.
Secondly, treat Sodium Phenoxide with dilute $HCl$ , and hence Phenol ( also known as carbolic acid ) is formed.
The reaction takes place according to the following mechanism:
Step 3: Structure of DDT:

Effects of DDT on environment are:
1) It is non-biodegradable in nature.
2) It is deposited and stored in fatty tissues.
Step 4: Two physical properties of carbolic acid:
1) It is a colourless, hygroscopic, crystalline solid with typical phenolic odour. On exposure to air and light, it turns pink due to partial oxidation.
2) It has low melting point, but has higher boiling point due to intermolecular hydrogen bonding.
Note:Dichlorodiphenyltrichloroethane, commonly known as DDT, is a colorless, tasteless, and almost odorless crystalline chemical compound, an organochlorine. Originally developed as an insecticide, it became infamous for its environmental impacts. Phenol is an aromatic organic compound with the molecular formula ${C_6}{H_5}OH$ . It is a white crystalline solid that is volatile.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




