
Carbocations may be stabilised by:
(A) $\pi $- bonds only at phenylic position
(B) $\pi $- bonds only at vinylic position
(C) $\pi $- bonds at allylic and benzylic position also
(D) -$I$ effect.
Answer
576k+ views
Hint: The stabilisation of the carbocations takes place by the neighbouring carbon atoms (having electron pairs) imparting some of the basic phenomenon as resonance effect, inductive effects, etc.
Complete step by step solution:
Let us study about the carbocations and the stabilisation criteria. Carbocations are the most reactive intermediate as positive charge is present on them attracts the electron groups from the reacting environment. They may be stabilised by,
1. Neighbouring carbon atoms (+I effect and hyperconjugation)- Alkyl groups are electron releasing groups through inductive effect, thus stabilising the carbocation. Hyperconjugation also stabilizes the carbocation by donating electrons from C-H sigma bond to the empty p orbital.
2. Neighbouring carbon-carbon multiple bonds (resonance effect)- Double bonds are rich in electrons in nature. The electron cloud present on $\pi -bond$ can donate electrons to the carbocationic carbon by resonance effect. This is called delocalisation of $\pi -electrons$.
3. Adjacent lone pairs (resonance effect)- The neighbouring atom donates the lone pair of electrons to the carbocation. Here the formation of double bonds takes place thus, it is also called as $\pi -donation$. In short, carbocation has a positive charge and thus can be stabilised when the electron donating group is attached to it. Now, seeing towards the given illustration, $\pi $- bonds at allylic and benzylic position also will stabilise the carbocation by resonance effects; then $\pi $- bonds only at phenylic position and $\pi $- bonds only at vinylic position.
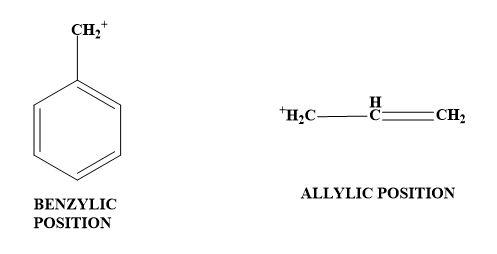
Therefore, option (C) is the correct answer.
Note: Option (D) can never be the answer as -I effect cannot stabilise the carbocation. Also, for option (A) and (B); the carbocation cannot be stabilised by the presence of $\pi $-bonds at phenylic position and vinylic position. And even if they do, the effect of stabilisation will be more if $\pi $-bond is present in an allylic and benzylic position.
Complete step by step solution:
Let us study about the carbocations and the stabilisation criteria. Carbocations are the most reactive intermediate as positive charge is present on them attracts the electron groups from the reacting environment. They may be stabilised by,
1. Neighbouring carbon atoms (+I effect and hyperconjugation)- Alkyl groups are electron releasing groups through inductive effect, thus stabilising the carbocation. Hyperconjugation also stabilizes the carbocation by donating electrons from C-H sigma bond to the empty p orbital.
2. Neighbouring carbon-carbon multiple bonds (resonance effect)- Double bonds are rich in electrons in nature. The electron cloud present on $\pi -bond$ can donate electrons to the carbocationic carbon by resonance effect. This is called delocalisation of $\pi -electrons$.
3. Adjacent lone pairs (resonance effect)- The neighbouring atom donates the lone pair of electrons to the carbocation. Here the formation of double bonds takes place thus, it is also called as $\pi -donation$. In short, carbocation has a positive charge and thus can be stabilised when the electron donating group is attached to it. Now, seeing towards the given illustration, $\pi $- bonds at allylic and benzylic position also will stabilise the carbocation by resonance effects; then $\pi $- bonds only at phenylic position and $\pi $- bonds only at vinylic position.

Therefore, option (C) is the correct answer.
Note: Option (D) can never be the answer as -I effect cannot stabilise the carbocation. Also, for option (A) and (B); the carbocation cannot be stabilised by the presence of $\pi $-bonds at phenylic position and vinylic position. And even if they do, the effect of stabilisation will be more if $\pi $-bond is present in an allylic and benzylic position.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




