
$CaOC{{l}_{2}}$ (bleaching powder) has two types of chlorine with different oxidation numbers. The sum of the oxidation numbers is ?
Answer
597k+ views
Hint: Draw the structure of $CaOC{{l}_{2}}$.$CaOC{{l}_{2}}$ chemical formula is also represented as $Ca(OCl)Cl$.Then assign the oxidation number for the known elements. The two chlorine atoms have different oxidation states. Consider the ions that will form if the 2 bonds with calcium breaks.
Complete step by step solution:
The chemical name of $CaOC{{l}_{2}}$(Bleaching powder) is Calcium hypochlorite. To find the oxidation number of Cl atoms in this compound, first we have to understand the structure of $CaOC{{l}_{2}}$.
So, let us take a look at its structure.
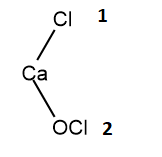
You can notice that the 3 atoms are not directly attached to the calcium atom. A better depiction of the chemical formula of $CaOC{{l}_{2}}$ would be $Ca(OCl)Cl$ . In this compound, we can see that one chlorine atom is directly attached to the calcium atom. Whereas, the other chlorine atom is actually attached to oxygen, which is in turn attached to calcium atom. It is therefore obvious that the two chlorine atoms have different oxidation numbers.
1. To find the oxidation number of the chlorine atoms, we will first consider the ions that will form if the 2 bonds with calcium breaks. The ions will be, $C{{l}^{-}}\text{ and (}OCl{{)}^{-}}$.
2. So we can now find the oxidation number of both chlorine atoms. The first chlorine atom will thus have an oxidation number of -1.
3. The second chlorine atom will have an oxidation number of +1. This is because oxygen has a -2 charge and the net charge of the ion formed is -1.
Therefore, sum of oxidation numbers = (-1) + 1 = 0.
Note: Do not make the structure in such a way that all the 3 atoms are directly bonded with the calcium atom. Such a structure is wrong. It would be better to use the chemical formula as $Ca(OCl)Cl$ instead of $CaOC{{l}_{2}}$ to avoid any confusion.
Complete step by step solution:
The chemical name of $CaOC{{l}_{2}}$(Bleaching powder) is Calcium hypochlorite. To find the oxidation number of Cl atoms in this compound, first we have to understand the structure of $CaOC{{l}_{2}}$.
So, let us take a look at its structure.

You can notice that the 3 atoms are not directly attached to the calcium atom. A better depiction of the chemical formula of $CaOC{{l}_{2}}$ would be $Ca(OCl)Cl$ . In this compound, we can see that one chlorine atom is directly attached to the calcium atom. Whereas, the other chlorine atom is actually attached to oxygen, which is in turn attached to calcium atom. It is therefore obvious that the two chlorine atoms have different oxidation numbers.
1. To find the oxidation number of the chlorine atoms, we will first consider the ions that will form if the 2 bonds with calcium breaks. The ions will be, $C{{l}^{-}}\text{ and (}OCl{{)}^{-}}$.
2. So we can now find the oxidation number of both chlorine atoms. The first chlorine atom will thus have an oxidation number of -1.
3. The second chlorine atom will have an oxidation number of +1. This is because oxygen has a -2 charge and the net charge of the ion formed is -1.
Therefore, sum of oxidation numbers = (-1) + 1 = 0.
Note: Do not make the structure in such a way that all the 3 atoms are directly bonded with the calcium atom. Such a structure is wrong. It would be better to use the chemical formula as $Ca(OCl)Cl$ instead of $CaOC{{l}_{2}}$ to avoid any confusion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




