
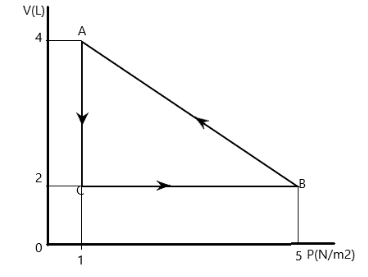
Calculate the work done for $ B \to A $
(A) $ 6 \times {10^{ - 3}}J $
(B) $ 12 \times {10^{ - 3}}J $
(C) $ 3 \times {10^{ - 3}}J $
(D) $ 4 \times {10^{ - 3}}J $

Answer
568.5k+ views
Hint: from B to A, observe that the pressure changes linearly. Generally work done can be defined as the integration of pressure with respect to volume through the path taken.
Formula used : In this solution we will be using the following formulae;
$ W = \int {PdV} $ where $ W $ is the work done on a gas, $ P $ is the pressure of the gas and $ V $ is the volume of the gas.
Complete step by step answer:
To calculate the work done, we note that the pressure is linearly changing with volume, hence, we can model it as an equation of a straight line as in
$ x = my + c $
Then, $ P = mV + c $
Now, from the diagram, we have that at volume equal to 2 litres (which is $ 2 \times {10^{ - 3}}{m^3} $ ), pressure is $ 5N/{m^2} $ .
Hence, $ 5 = m\left( {2 \times {{10}^{ - 3}}} \right) + c $
And similarly from diagram, we also get
$ 1 = m\left( {4 \times {{10}^{ - 3}}} \right) + c $
Solving the two equations simultaneously, we get that
$ m = - 2 \times {10^3} $ and $ c = 9 $
Hence, $ P = - 2 \times {10^3}V + 9 $
Now, the work done can be defined as
$ W = \int {PdV} $ where $ W $ is the work done on a gas, $ P $ is the pressure of the gas and $ V $ is the volume of the gas.
So by inserting expression and integrating, we have
$ W = \int_{{V_B}}^{{V_A}} {PdV} = \int_{2 \times {{10}^{ - 3}}}^{4 \times {{10}^{ - 3}}} {\left( { - 2 \times {{10}^3}V + 9} \right)dV} $
Hence, $ W = \left[ { - {{10}^3}{V^2} + 9V} \right]_{2 \times {{10}^{ - 3}}}^{4 \times {{10}^{ - 3}}} $
Hence, by carrying out the definite integral, we have that
$ W = - 6 \times {10^{ - 3}}J $
We can neglect the negative sign as it only means that work is done by the gas from B to A, hence, we get
$ W = 6 \times {10^{ - 3}}J $
Hence, the correct option is A.
Note:
For clarity, we can write the equation of a straight line as $ x = my + c $ . This is in contrast to the well known format of $ y = mx + c $ . However, it can be written the other way around too, which can be proven as in;
$ y = mx + c $
$ x = \dfrac{{y - c}}{m} = \dfrac{y}{m} - \dfrac{c}{m} $
But since $ m $ and c are constants, we have that $ \dfrac{1}{m} $ and $ \dfrac{c}{m} $ are constant. And there is the slope and intercept of the graph if $ x $ is considered the dependent variable. Hence
$ x = {m_1}y - {c_1} $ .
Formula used : In this solution we will be using the following formulae;
$ W = \int {PdV} $ where $ W $ is the work done on a gas, $ P $ is the pressure of the gas and $ V $ is the volume of the gas.
Complete step by step answer:
To calculate the work done, we note that the pressure is linearly changing with volume, hence, we can model it as an equation of a straight line as in
$ x = my + c $
Then, $ P = mV + c $
Now, from the diagram, we have that at volume equal to 2 litres (which is $ 2 \times {10^{ - 3}}{m^3} $ ), pressure is $ 5N/{m^2} $ .
Hence, $ 5 = m\left( {2 \times {{10}^{ - 3}}} \right) + c $
And similarly from diagram, we also get
$ 1 = m\left( {4 \times {{10}^{ - 3}}} \right) + c $
Solving the two equations simultaneously, we get that
$ m = - 2 \times {10^3} $ and $ c = 9 $
Hence, $ P = - 2 \times {10^3}V + 9 $
Now, the work done can be defined as
$ W = \int {PdV} $ where $ W $ is the work done on a gas, $ P $ is the pressure of the gas and $ V $ is the volume of the gas.
So by inserting expression and integrating, we have
$ W = \int_{{V_B}}^{{V_A}} {PdV} = \int_{2 \times {{10}^{ - 3}}}^{4 \times {{10}^{ - 3}}} {\left( { - 2 \times {{10}^3}V + 9} \right)dV} $
Hence, $ W = \left[ { - {{10}^3}{V^2} + 9V} \right]_{2 \times {{10}^{ - 3}}}^{4 \times {{10}^{ - 3}}} $
Hence, by carrying out the definite integral, we have that
$ W = - 6 \times {10^{ - 3}}J $
We can neglect the negative sign as it only means that work is done by the gas from B to A, hence, we get
$ W = 6 \times {10^{ - 3}}J $
Hence, the correct option is A.
Note:
For clarity, we can write the equation of a straight line as $ x = my + c $ . This is in contrast to the well known format of $ y = mx + c $ . However, it can be written the other way around too, which can be proven as in;
$ y = mx + c $
$ x = \dfrac{{y - c}}{m} = \dfrac{y}{m} - \dfrac{c}{m} $
But since $ m $ and c are constants, we have that $ \dfrac{1}{m} $ and $ \dfrac{c}{m} $ are constant. And there is the slope and intercept of the graph if $ x $ is considered the dependent variable. Hence
$ x = {m_1}y - {c_1} $ .
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE




