
$But - 2 - ene$ on reaction with alkaline $KMn{O_4}$ at elevated temperature followed
by acidification will give:
(A) One molecule of $C{H_3}CHO$ and one molecule of $C{H_3}COOH$
(B)
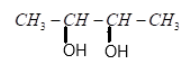
(C) $2$ molecules of $C{H_3}COOH$
(D) $2$molecules of $C{H_3}CHO$
Answer
569.4k+ views
Hint: As we know that alkene can react with cold, acidic, and alkaline potassium permanganate and form different products so alkaline $KMn{O_4}$ being an oxidising agent when added to an alkene it results in the ketonic or acidic compounds formation.
Complete Step by step answer: As we know that the carbon-carbon double bond in alkene reacts with the alkaline potassium permanganate followed by acidification and results into a ketone or acidic compound formation. Alkene on reaction with cold, dilute or aqueous solution of potassium permanganate also known as Beyer’s reagent, produces vicinal glycols.
So, $But - 2 - ene$ on reaction with potassium permanganate oxidises the compound and results in the formation of two molecules of Ethanoic acid.
$C{H_3} - CH = CH - C{H_3}\xrightarrow{{KMn{O_4}}}2\;C{H_3}COOH$
We can show this reaction as:
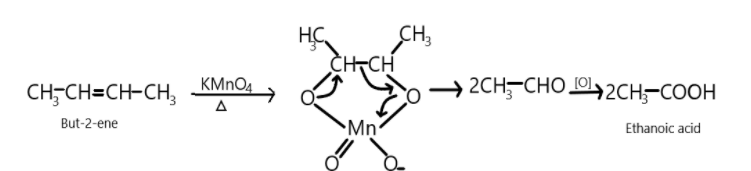
So first alkene reacts with potassium permanganate and breakdown or double bonds take place resulting into the formation of two aldehydic compounds which on further oxidation produces two molecules of ethanoic acid which is the main product in the following reaction.
Therefore the correct answer is (C).
Note: potassium permanganate is a crystalline solid which is purple-black in colour and discolouration of potassium permanganate solution is used as a test for unsaturation. Alkaline potassium permanganate can be used to identify whether the organic compound is an alkene or an alkane.
Complete Step by step answer: As we know that the carbon-carbon double bond in alkene reacts with the alkaline potassium permanganate followed by acidification and results into a ketone or acidic compound formation. Alkene on reaction with cold, dilute or aqueous solution of potassium permanganate also known as Beyer’s reagent, produces vicinal glycols.
So, $But - 2 - ene$ on reaction with potassium permanganate oxidises the compound and results in the formation of two molecules of Ethanoic acid.
$C{H_3} - CH = CH - C{H_3}\xrightarrow{{KMn{O_4}}}2\;C{H_3}COOH$
We can show this reaction as:

So first alkene reacts with potassium permanganate and breakdown or double bonds take place resulting into the formation of two aldehydic compounds which on further oxidation produces two molecules of ethanoic acid which is the main product in the following reaction.
Therefore the correct answer is (C).
Note: potassium permanganate is a crystalline solid which is purple-black in colour and discolouration of potassium permanganate solution is used as a test for unsaturation. Alkaline potassium permanganate can be used to identify whether the organic compound is an alkene or an alkane.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




