
Bromination of 2- butene dioic acid gives:
A. (2R,3S)- 2, 3 dibromo succinic acid
B. (2R,3R)- 23 dibromo succinic acid
C. A mixture of (2R,3R) and (2S,3S) – 2, 3 dibromo succinic acid
D. (2S,3S)-2,3 dibromo succinic acid
Answer
585k+ views
Hint: Treatment of alkenes with bromine gives vicinal dibromides. Attack of the alkene in bromine gives the bromonium ion, which is attacked at the backside by bromide ion to give the trans dibromo product. Note that the bromines are delivered to opposite sides of the alkenes. The two atoms that form a new bond to carbon add to the opposite faces of the alkene. This is a stereo selective reaction.
Complete step by step answer:
Alkenes react with bromine in the cold with organic solvent. The bond breaks, a bromine atom becomes attached with each carbon. The bromine loses its original red brown color to give a colorless liquid. Bromination is the example of electrophilic addition. The bromine is a very polarized molecule and the approaching pi bond in the alkene induces a dipole in the bromine molecule.
Here the configuration of Bromination of 2- butene dioic acid is (2R,3S)- 2, 3 dibromo succinic acid or (2S, 3R)-2,3- dibromo succinic acid.
The reaction is given below:
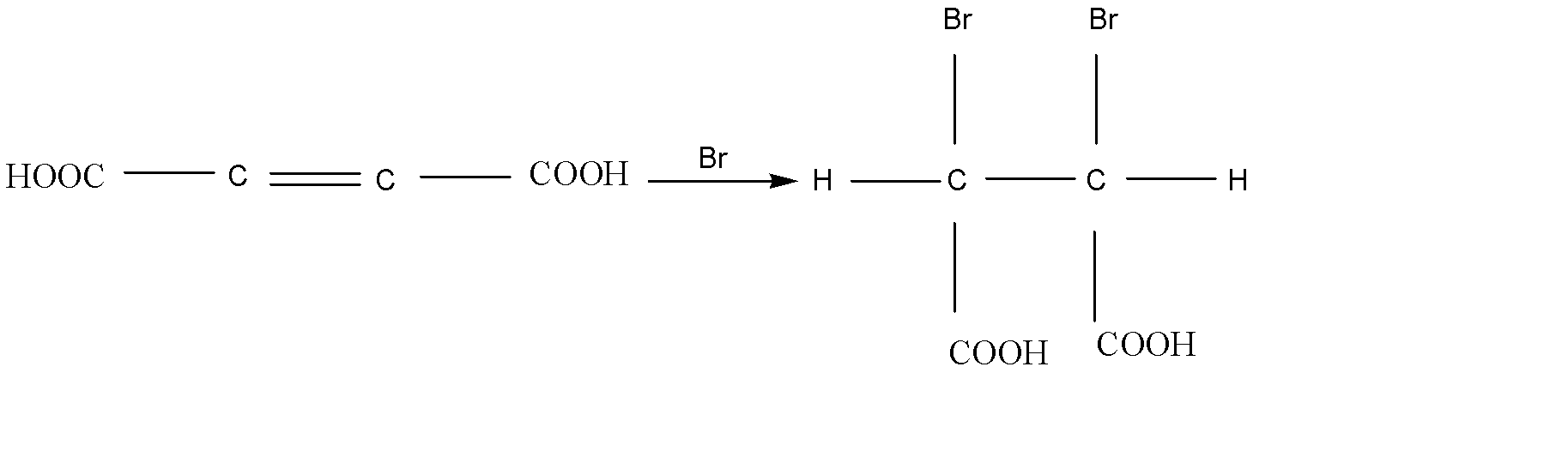
Therefore, option (A) is the correct answer.
Note: Consider using the reaction for this species. All reactions involving this species. A general reaction form is also available. If Bromination occurs on an alkene adjacent to an aromatic ring, some products that appear to have been produced from syn Bromination observed. This is particularly true in any case where a long lived, stable carbocation can be formed.
Complete step by step answer:
Alkenes react with bromine in the cold with organic solvent. The bond breaks, a bromine atom becomes attached with each carbon. The bromine loses its original red brown color to give a colorless liquid. Bromination is the example of electrophilic addition. The bromine is a very polarized molecule and the approaching pi bond in the alkene induces a dipole in the bromine molecule.
Here the configuration of Bromination of 2- butene dioic acid is (2R,3S)- 2, 3 dibromo succinic acid or (2S, 3R)-2,3- dibromo succinic acid.
The reaction is given below:

Therefore, option (A) is the correct answer.
Note: Consider using the reaction for this species. All reactions involving this species. A general reaction form is also available. If Bromination occurs on an alkene adjacent to an aromatic ring, some products that appear to have been produced from syn Bromination observed. This is particularly true in any case where a long lived, stable carbocation can be formed.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




