
Why is $BrF_4^ - $ square planar, whereas $BF_4^ - $ is tetrahedral?
Answer
461.1k+ views
Hint: The geometry of square planar molecules describes the stereochemistry adopted by chemical compounds. The d-orbital splitting diagram for square planar $\left( {{D_{4h}}}
\right)$transition metal complexes is derived from the general octahedral $\left( {{O_h}} \right)$splitting diagram.
Complete answer:
In this question, bromine has $7$ valence electrons in its ground state electronic configuration whereas boron has only $3$ valence electrons in its ground state electronic configuration as given below:
For $Br$,
$\left[ {Ar} \right]4{s^2}3{d^{10}}4{p^5}$
For $B$,
$\left[ {He} \right]2{s^2}2{p^1}$
In these electronic configurations, we can see that the valence shell of bromine contains $7$ electrons, $2$ from the $s$ subshell and $5$ in the $p$ subshell.
In the first case when $Br$ is the central atom bonded with four $F$ atoms, the four of the seven electrons of bromine form a bond with $F$ atom. Here this shows that three electrons do not take part in bond formation. As there is a negative charge in the compound this indicates the additional electrons which will be paired with one of the three lone electrons.
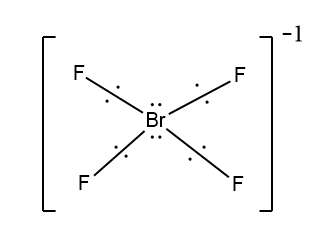
Here you can see that four \[F\] will align in a square plane and the lone pairs are on either side of the plane.
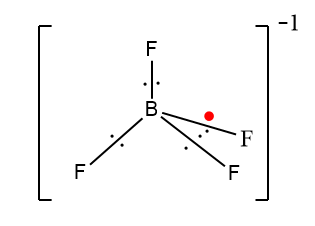
In the case of $B{F_4}^ - $, boron only has three valence electrons, so three $F$ will bond with boron and the fourth $F$will occupy the open $p$ orbital of $B$. Here the extra electron shown in red is not present in the ground state configurations that provide the negative charge.
Thus, by these diagrams it is clear that $BrF_4^ - $ has square planar and $BF_4^ - $ has a tetrahedral shape.
Note:
In square planar, the constituent atoms surround the central atom which form the corners of a square on the same plane. Whereas in the tetrahedral, on the centre of the four substituents, there the central atom forming the corners of the tetrahedron.
\right)$transition metal complexes is derived from the general octahedral $\left( {{O_h}} \right)$splitting diagram.
Complete answer:
In this question, bromine has $7$ valence electrons in its ground state electronic configuration whereas boron has only $3$ valence electrons in its ground state electronic configuration as given below:
For $Br$,
$\left[ {Ar} \right]4{s^2}3{d^{10}}4{p^5}$
For $B$,
$\left[ {He} \right]2{s^2}2{p^1}$
In these electronic configurations, we can see that the valence shell of bromine contains $7$ electrons, $2$ from the $s$ subshell and $5$ in the $p$ subshell.
In the first case when $Br$ is the central atom bonded with four $F$ atoms, the four of the seven electrons of bromine form a bond with $F$ atom. Here this shows that three electrons do not take part in bond formation. As there is a negative charge in the compound this indicates the additional electrons which will be paired with one of the three lone electrons.

Here you can see that four \[F\] will align in a square plane and the lone pairs are on either side of the plane.
In the case of $B{F_4}^ - $, boron only has three valence electrons, so three $F$ will bond with boron and the fourth $F$will occupy the open $p$ orbital of $B$. Here the extra electron shown in red is not present in the ground state configurations that provide the negative charge.

Thus, by these diagrams it is clear that $BrF_4^ - $ has square planar and $BF_4^ - $ has a tetrahedral shape.
Note:
In square planar, the constituent atoms surround the central atom which form the corners of a square on the same plane. Whereas in the tetrahedral, on the centre of the four substituents, there the central atom forming the corners of the tetrahedron.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




