
Boron nitride can be represented by the given structure. The structure of ${\text{BN}}$ is similar to:
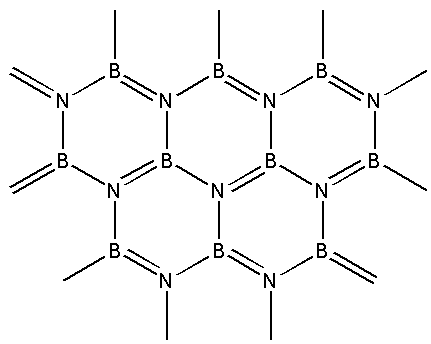
A) Graphite
B) Diamond
C) Benzene
D) Pyridine

Answer
561k+ views
Hint: Boron nitride consists of boron and nitrogen atoms. Boron nitride is amorphous in nature. It exists in hexagonal, cubic and wurtzite form. We are given a hexagonal form of boron nitride. To solve this we must know about the structures of graphite, diamond, benzene and pyridine.
Complete step-by-step answer:
We are given the structure of boron nitride i.e. ${\text{BN}}$ as follows:
From the structure, we can see that boron nitride consists of boron and nitrogen atoms. Boron nitride is amorphous in nature. It exists in hexagonal, cubic and wurtzite form. We are given a hexagonal form of boron nitride.
In the hexagonal structure of boron nitride, the boron and nitrogen atoms are held together by weak van der Waals forces. The structure is a layered structure. Within each layer, strong covalent bonds exist between the boron and nitrogen atoms. This structure of boron nitride is similar to that of graphite.
Graphite is an allotrope of carbon. In graphite, the carbon atoms are present in a hexagonal arrangement. The hexagonal arrangement of carbon atoms forms layers which have weak van der Waals forces between them. Within each layer, strong covalent bonds exist between the carbon atoms. Thus, the structure of ${\text{BN}}$ is similar to graphite.
Thus, the correct option is (A) graphite.
Note: Also, the specific heat capacity of water is high. Thus, the presence of large amounts of water modifies the climate of the nearby lands. This makes the adjacent lands warmer in winter and cooler in summer. Thus, as Mumbai and Chennai lie near sea bodies the temperature in these areas does not fall to a very low level.
Complete step-by-step answer:
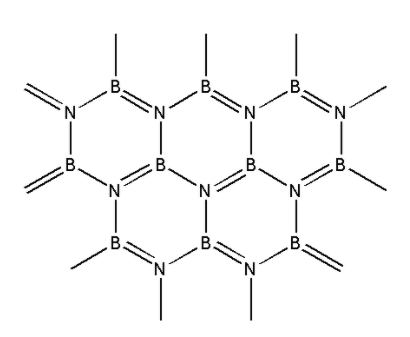
We are given the structure of boron nitride i.e. ${\text{BN}}$ as follows:

From the structure, we can see that boron nitride consists of boron and nitrogen atoms. Boron nitride is amorphous in nature. It exists in hexagonal, cubic and wurtzite form. We are given a hexagonal form of boron nitride.
In the hexagonal structure of boron nitride, the boron and nitrogen atoms are held together by weak van der Waals forces. The structure is a layered structure. Within each layer, strong covalent bonds exist between the boron and nitrogen atoms. This structure of boron nitride is similar to that of graphite.
Graphite is an allotrope of carbon. In graphite, the carbon atoms are present in a hexagonal arrangement. The hexagonal arrangement of carbon atoms forms layers which have weak van der Waals forces between them. Within each layer, strong covalent bonds exist between the carbon atoms. Thus, the structure of ${\text{BN}}$ is similar to graphite.
Thus, the correct option is (A) graphite.
Note: Also, the specific heat capacity of water is high. Thus, the presence of large amounts of water modifies the climate of the nearby lands. This makes the adjacent lands warmer in winter and cooler in summer. Thus, as Mumbai and Chennai lie near sea bodies the temperature in these areas does not fall to a very low level.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




