
How many bonds can Carbon form? Why is this important?
Answer
565.2k+ views
Hint:In order to find the number of bonds Carbon can form, we must first know the atomic number of carbon atoms. On knowing the atomic number, we can find the valence electron present in it. By knowing the valence electrons, we can find the number of bonds that Carbon can form.
Complete answer:
Let us first know about Carbon. Carbon is a chemical element with an atomic number of 6 and is represented by the chemical symbol of C. It will have an electronic configuration of $1{s^2}$ $2{s^2}$ $2{p^2}$ . The carbon will be having four valence electrons. Carbon atoms can form four chemical bonds as there are four valence electrons. The chemical bond formation takes place in order to satisfy the valency of the atom so that it can achieve a stable electronic configuration. Therefore, the carbon will form a Covalent bond with other atoms by mutual sharing of the electrons.
Let us consider an example of a methane molecule. In methane molecules, the carbon will be having a valency of 4 and it will form a covalent bond with four hydrogen atoms whose valency is 1. The chemical bond formation will take place by the mutual sharing of electrons. So that both Carbon and Hydrogen will attain a stable electronic configuration.
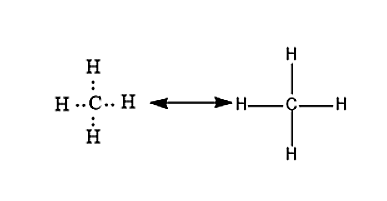
Note:
We have to remember that not only the formation of the covalent bond but also formation of other bonds takes place in different atoms they are
- Ionic bond
- Metallic bond
- Hydrogen bond
Complete answer:
Let us first know about Carbon. Carbon is a chemical element with an atomic number of 6 and is represented by the chemical symbol of C. It will have an electronic configuration of $1{s^2}$ $2{s^2}$ $2{p^2}$ . The carbon will be having four valence electrons. Carbon atoms can form four chemical bonds as there are four valence electrons. The chemical bond formation takes place in order to satisfy the valency of the atom so that it can achieve a stable electronic configuration. Therefore, the carbon will form a Covalent bond with other atoms by mutual sharing of the electrons.
Let us consider an example of a methane molecule. In methane molecules, the carbon will be having a valency of 4 and it will form a covalent bond with four hydrogen atoms whose valency is 1. The chemical bond formation will take place by the mutual sharing of electrons. So that both Carbon and Hydrogen will attain a stable electronic configuration.

Note:
We have to remember that not only the formation of the covalent bond but also formation of other bonds takes place in different atoms they are
- Ionic bond
- Metallic bond
- Hydrogen bond
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




