
Bond angle present in the methane ($CH_{ 4 }$) molecule is:
A.105$^{ 0 }$
B.107$^{ 0 }$
C.109$^{ 0 }$
D.110$^{ 0 }$
Answer
600k+ views
Hint: Methane molecule has ${ sp }^{ 3 }$ hybridization. Now try to figure out the value for the standard bond angle in this hybridization.
Complete step by step answer:
-Let’s talk about the $CH_{ 4 }$ molecule. This is basically a combination of 1 carbon atom and 4 hydrogen atoms. However, to form this compound the central atom carbon has to complete its octet. It has 4 valence electrons and it obtains 4 more electrons from 4 hydrogen atoms. Hence, by sharing electrons between carbon and hydrogen there is a formation of a covalent bond.
-Now, we will discuss the hybridization of methane, the carbon here is ${ sp }^{ 3 }$ hybridized because one 2s orbital and three 2p orbitals in the valence shell of carbon combine to form four ${ sp }^{ 3 }$ hybrid orbitals that are of equal energy and also have equal shape. Further, four H atoms also use these four ${ sp }^{ 3 }$ hybrid orbitals of carbon to form four sigma bonds. It finally leads to the formation of the methane molecule.
-We have discussed the hybridization process. Now, determining the molecular geometry of methane should be easier for us. In methane, the four hybrid orbitals are located in such a manner so that they can decrease the force of repulsion between them. Hence, $CH_{ 4 }$ acquires a tetrahedral shape.
Hence, the ${ sp }^{ 3 }$ hybrid orbitals have a bond angle of 109$^{ 0 }$28’. We can roughly assume it to be 109$^{ 0 }$.
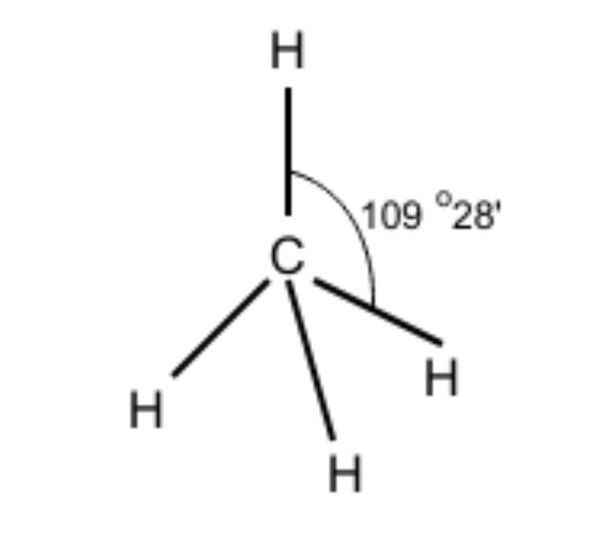
Therefore, we can conclude that the correct answer to this question is option C.
Note:
All angles are the same in this compound with a bond length between all C-H bonds equal to 1.09 Angstrom. All the outer atoms are the same - the same dipoles, and that the dipole moments are in the same direction - towards the carbon atom, the overall molecule becomes non-polar. Therefore, methane has nonpolar bonds and is nonpolar overall.
Complete step by step answer:
-Let’s talk about the $CH_{ 4 }$ molecule. This is basically a combination of 1 carbon atom and 4 hydrogen atoms. However, to form this compound the central atom carbon has to complete its octet. It has 4 valence electrons and it obtains 4 more electrons from 4 hydrogen atoms. Hence, by sharing electrons between carbon and hydrogen there is a formation of a covalent bond.
-Now, we will discuss the hybridization of methane, the carbon here is ${ sp }^{ 3 }$ hybridized because one 2s orbital and three 2p orbitals in the valence shell of carbon combine to form four ${ sp }^{ 3 }$ hybrid orbitals that are of equal energy and also have equal shape. Further, four H atoms also use these four ${ sp }^{ 3 }$ hybrid orbitals of carbon to form four sigma bonds. It finally leads to the formation of the methane molecule.
-We have discussed the hybridization process. Now, determining the molecular geometry of methane should be easier for us. In methane, the four hybrid orbitals are located in such a manner so that they can decrease the force of repulsion between them. Hence, $CH_{ 4 }$ acquires a tetrahedral shape.
Hence, the ${ sp }^{ 3 }$ hybrid orbitals have a bond angle of 109$^{ 0 }$28’. We can roughly assume it to be 109$^{ 0 }$.

Therefore, we can conclude that the correct answer to this question is option C.
Note:
All angles are the same in this compound with a bond length between all C-H bonds equal to 1.09 Angstrom. All the outer atoms are the same - the same dipoles, and that the dipole moments are in the same direction - towards the carbon atom, the overall molecule becomes non-polar. Therefore, methane has nonpolar bonds and is nonpolar overall.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




