
What is the bond angle in a tetrahedral molecule?
Answer
503.4k+ views
Hint: According to the VSEPR theory, each atom in a molecule will obtain a shape that minimises repulsion between electrons in that atom's valence shell. It's essentially a model for predicting molecular geometry. The bonding and molecular geometry of organic molecules and polyatomic ions are the main focus of VSEPR models.
Complete answer:
As we know, tetra means four and hedral means a solid face, therefore tetrahedral means "having four faces." When four bonds are all linked to one core atom and there are no lone electron pairs, this shape is formed.
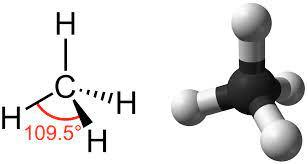
A tetrahedral molecule is made up $ 4 $ equally spaced $ s{p^3} $ hybrid orbitals forming bond angles of $ {109.5^ \circ } $
Example of a tetrahedral molecule is methane.
The four equivalent bonds ( $ C - H $ ) point in four geometrically equivalent directions in three dimensions which can be corresponded to the four corners of a tetrahedron as hydrogen, centred on the carbon atom.
Additional Information:
The VSEPR model accurately predicts the $ 3 $ -D shape of molecules and ions, but it fails to provide any detailed information on bond length or bond structure.
VSEPR models are based on the idea that electrons revolving around a core atom will arrange themselves to minimise repulsion, dictating the molecule's shape.
It can predict the form of almost every compound with a centre atom.
Note:
When counting bond angles, always use shapes above geometries of the given molecule because bond angles are always between bonds, not between lone pairs. Shape primarily examines real bonds, whereas geometry considers the total arrangement of a molecule, including bonds and lone pairs.
Complete answer:
As we know, tetra means four and hedral means a solid face, therefore tetrahedral means "having four faces." When four bonds are all linked to one core atom and there are no lone electron pairs, this shape is formed.
A tetrahedral molecule is made up $ 4 $ equally spaced $ s{p^3} $ hybrid orbitals forming bond angles of $ {109.5^ \circ } $
Example of a tetrahedral molecule is methane.
The four equivalent bonds ( $ C - H $ ) point in four geometrically equivalent directions in three dimensions which can be corresponded to the four corners of a tetrahedron as hydrogen, centred on the carbon atom.

Additional Information:
The VSEPR model accurately predicts the $ 3 $ -D shape of molecules and ions, but it fails to provide any detailed information on bond length or bond structure.
VSEPR models are based on the idea that electrons revolving around a core atom will arrange themselves to minimise repulsion, dictating the molecule's shape.
It can predict the form of almost every compound with a centre atom.
Note:
When counting bond angles, always use shapes above geometries of the given molecule because bond angles are always between bonds, not between lone pairs. Shape primarily examines real bonds, whereas geometry considers the total arrangement of a molecule, including bonds and lone pairs.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

Which out of the following hydrocarbons undergo addition class 11 chemistry CBSE




