
Bleaching powder contains:
a.) CaO and $C{{l}_{2}}$ molecules
b.) $C{{a}^{2+}}$ and $2OC{{l}^{-}}$ ions
c.) $C{{a}^{2+}},C{{l}^{-}}\text{ and OC}{{\text{l}}^{-}}$ ions
d.) $C{{a}^{2+}},{{O}^{2-}}$ ions, and $C{{l}_{2}}$ molecules
Answer
586.5k+ views
Hint: Chemical formula of bleaching powder is $Ca{{\left( OCl \right)}_{2}}$ with its chemical name as Calcium hypochlorite. Now just try to make its structure to find the species present in it.
Complete step by step answer:
Bleaching powder is a pale yellowish powder existing with a strong smell of chlorine.
It is soluble in water but due to the presence of impurities, we never observe a clear solution.
Bleaching powder contains calcium hydroxide, calcium chloride, and calcium hypochlorite which are blended and reduced to powder. The following is the reaction of preparation of bleaching powder: $2C{{l}_{2}}+2Ca{{\left( OH \right)}_{2}}\to Ca{{\left( OCl \right)}_{2}}+CaC{{l}_{2}}+2{{H}_{2}}O$
Bleaching powder is the chemical compound with the formula $Ca{{\left( OCl \right)}_{2}}$. Its structural formula shows the presence of $C{{a}^{2+}}$ and $2OC{{l}^{-}}$ ions.
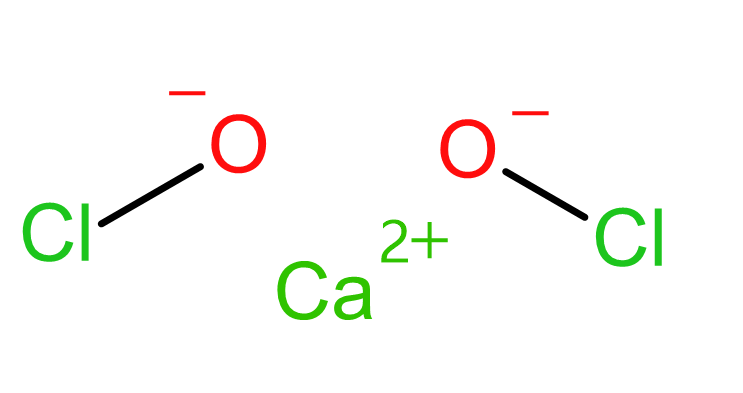
So, the correct answer is “Option B”.
Additional Information:
Calcium hypochlorite is stored dry and cold, away from any acid, organic materials, and metals. The hydrated form is safer to handle.
If mixed with an acid it releases highly toxic chlorine gas.
Note: We should also know the uses of bleaching powder -
It is used for bleaching dirty clothes in the laundry.
It is a strong oxidizing agent hence used as an oxidizer in many industries.
It is used as a disinfectant which is used for disinfecting water.
Complete step by step answer:
Bleaching powder is a pale yellowish powder existing with a strong smell of chlorine.
It is soluble in water but due to the presence of impurities, we never observe a clear solution.
Bleaching powder contains calcium hydroxide, calcium chloride, and calcium hypochlorite which are blended and reduced to powder. The following is the reaction of preparation of bleaching powder: $2C{{l}_{2}}+2Ca{{\left( OH \right)}_{2}}\to Ca{{\left( OCl \right)}_{2}}+CaC{{l}_{2}}+2{{H}_{2}}O$
Bleaching powder is the chemical compound with the formula $Ca{{\left( OCl \right)}_{2}}$. Its structural formula shows the presence of $C{{a}^{2+}}$ and $2OC{{l}^{-}}$ ions.

So, the correct answer is “Option B”.
Additional Information:
Calcium hypochlorite is stored dry and cold, away from any acid, organic materials, and metals. The hydrated form is safer to handle.
If mixed with an acid it releases highly toxic chlorine gas.
Note: We should also know the uses of bleaching powder -
It is used for bleaching dirty clothes in the laundry.
It is a strong oxidizing agent hence used as an oxidizer in many industries.
It is used as a disinfectant which is used for disinfecting water.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

The Equation xxx + 2 is Satisfied when x is Equal to Class 10 Maths

Which Country is Called "The Land of Festivals"?

What is Contraception List its four different methods class 10 biology CBSE




