
Binding of antigen to the antibody is through?
(a)electrostatic interactions
(b)covalent bonds
(c)disulphide bridges
(d)amide formation
Answer
593.4k+ views
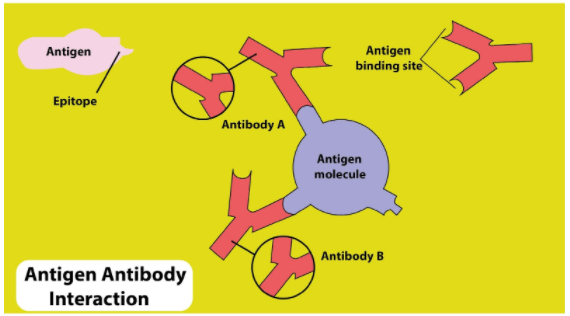
Hint: Each antigen causes the formation of a specific antibody. Antigen and antibody have complementary reactive sites that fit together like lock-key. Antibodies attach to antigens through poor chemical interactions, and bonding is basically non-covalent.
Complete answer:
Noncovalent interactions such as electrostatic interactions, hydrogen bonds, Van der Waals forces, and hydrophobic interactions are interactions through which antigens are bound to antibodies.
Additional Information:
Agglutination is the process in which antigens and antibodies combine. It is the fundamental reaction of the immune system in the body. By this process, our body is protected from complex foreign molecules, such as pathogens and their chemical toxins. In the blood, the antigens are particularly present and with high affinity bound by antibodies to form an antigen-antibody complex. The immune complex is then taken to cellular systems where it can be eradicated or deactivated. Non-covalent bonds between antibody and the antigen can also be moderated by interfacial water molecules. These indirect bonds can participate in the phenomenon of cross-reactivity, i.e. the recognition of various but similar antigens by a single antibody. Antigen and antibody interact through a high-affinity binding similar to lock and key mechanism. The altogether strength of the binding of an antibody to an antigen is termed its avidity for that antigen. Since antibodies are bivalent or polyvalent, this is the sum of the powers of individual antibody-antigen interactions. The stability of individual interaction between a single binding site on an antibody and its target epitope is termed the affinity of that interaction.
So the correct answer is ”electrostatic interactions”.
Note: The requirements of specificity and cross-reactivity of the antigen-antibody interaction are helpful in the clinical laboratory for diagnostic bases. One of the basic uses is the determination of ABO blood group and serological test against various pathogens.
Complete answer:
Noncovalent interactions such as electrostatic interactions, hydrogen bonds, Van der Waals forces, and hydrophobic interactions are interactions through which antigens are bound to antibodies.
Additional Information:
Agglutination is the process in which antigens and antibodies combine. It is the fundamental reaction of the immune system in the body. By this process, our body is protected from complex foreign molecules, such as pathogens and their chemical toxins. In the blood, the antigens are particularly present and with high affinity bound by antibodies to form an antigen-antibody complex. The immune complex is then taken to cellular systems where it can be eradicated or deactivated. Non-covalent bonds between antibody and the antigen can also be moderated by interfacial water molecules. These indirect bonds can participate in the phenomenon of cross-reactivity, i.e. the recognition of various but similar antigens by a single antibody. Antigen and antibody interact through a high-affinity binding similar to lock and key mechanism. The altogether strength of the binding of an antibody to an antigen is termed its avidity for that antigen. Since antibodies are bivalent or polyvalent, this is the sum of the powers of individual antibody-antigen interactions. The stability of individual interaction between a single binding site on an antibody and its target epitope is termed the affinity of that interaction.

So the correct answer is ”electrostatic interactions”.
Note: The requirements of specificity and cross-reactivity of the antigen-antibody interaction are helpful in the clinical laboratory for diagnostic bases. One of the basic uses is the determination of ABO blood group and serological test against various pathogens.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




