
Between 2-Methyl hexane and 2, 2-Dimethyl pentane which has a higher boiling point?
(a)- 2-Methyl hexane
(b)- 2, 2-Dimethyl pentane
(c)- Both have an equal boiling point
(d)- None of these
Answer
590.4k+ views
Hint: 2-Methyl hexane and 2, 2-Dimethyl pentane both are alkanes. And the boiling point of alkanes is based on the chain length and branching factor of the alkane. If the alkane has more branching then it has a low boiling point.
Complete step by step answer:
Alkanes are those compounds in which there are carbon and hydrogen atoms present and there is only a single bond. 2-Methyl hexane and 2, 2-Dimethyl pentane both are alkanes.
Among the alkanes, the chain having one to four carbon atoms are gases, the chain having five to seventeen carbon atoms are liquids, and the chain having eighteen or more carbon atoms are waxy solids. From this, we can say that as the number of carbon atoms or the length of chain increases the boiling point increases.
But this is not the same when there is branching in the alkane, as the branching in the alkane increases, the alkane will have the shape of a sphere and the length of the chain decreases, due to which the magnitude of van der Waal forces decreases. This causes a lowering in the boiling point.
So, the structure of 2-Methyl hexane is:
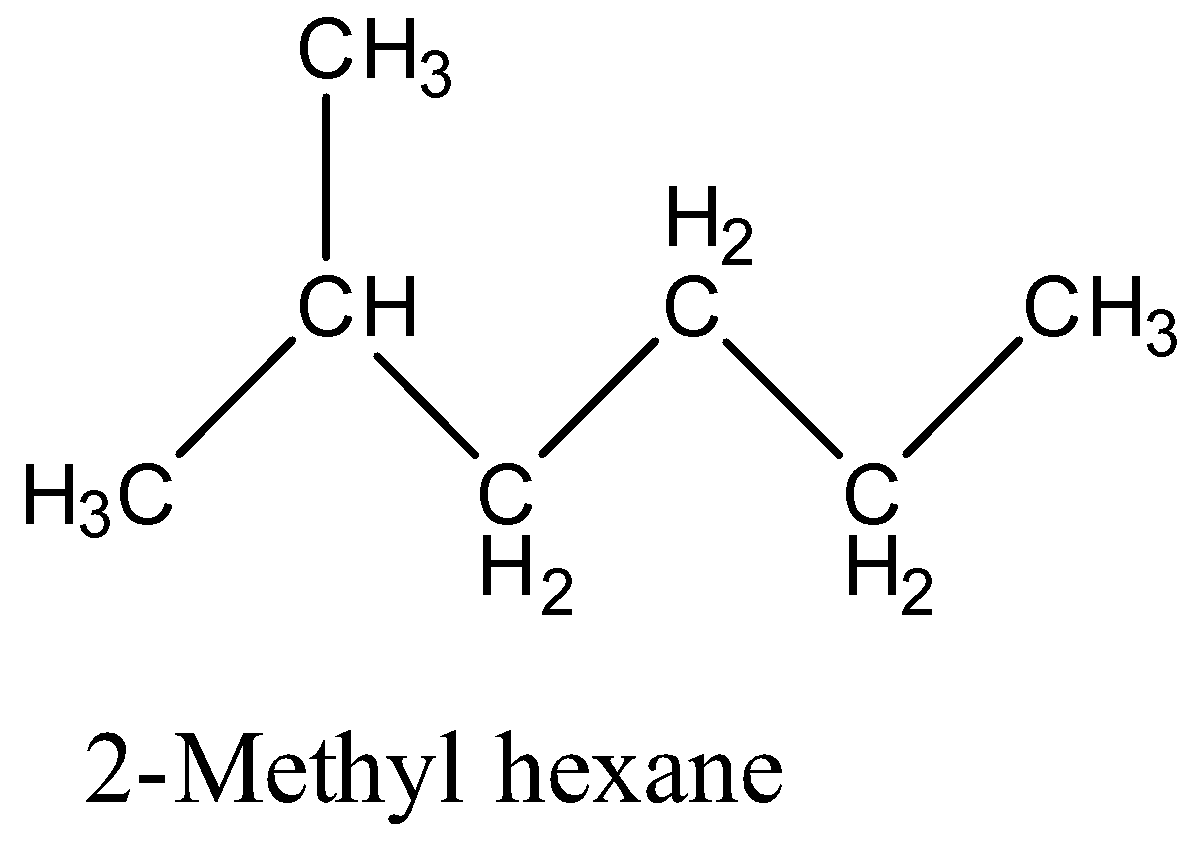
The structure of 2, 2-Dimethyl pentane is:

The 2, 2-Dimethyl pentane has two methyl groups in the branching form, and 2-Methyl hexane has one methyl group in branching. So, 2, 2-Dimethyl pentane has more branching than 2-Methyl hexane, therefore, 2-Methyl hexane has a higher boiling point than 2, 2-Dimethyl pentane.
Therefore, the correct answer is an option (a)- 2-Methyl hexane.
Note: As the number of carbon atoms in the chain increases the boiling point increases and generally 20-30K is increased when a carbon atom or methyl group ($-C{{H}_{2}}$) is added to the previous one.
Complete step by step answer:
Alkanes are those compounds in which there are carbon and hydrogen atoms present and there is only a single bond. 2-Methyl hexane and 2, 2-Dimethyl pentane both are alkanes.
Among the alkanes, the chain having one to four carbon atoms are gases, the chain having five to seventeen carbon atoms are liquids, and the chain having eighteen or more carbon atoms are waxy solids. From this, we can say that as the number of carbon atoms or the length of chain increases the boiling point increases.
But this is not the same when there is branching in the alkane, as the branching in the alkane increases, the alkane will have the shape of a sphere and the length of the chain decreases, due to which the magnitude of van der Waal forces decreases. This causes a lowering in the boiling point.
So, the structure of 2-Methyl hexane is:

The structure of 2, 2-Dimethyl pentane is:

The 2, 2-Dimethyl pentane has two methyl groups in the branching form, and 2-Methyl hexane has one methyl group in branching. So, 2, 2-Dimethyl pentane has more branching than 2-Methyl hexane, therefore, 2-Methyl hexane has a higher boiling point than 2, 2-Dimethyl pentane.
Therefore, the correct answer is an option (a)- 2-Methyl hexane.
Note: As the number of carbon atoms in the chain increases the boiling point increases and generally 20-30K is increased when a carbon atom or methyl group ($-C{{H}_{2}}$) is added to the previous one.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




