
Benzenesulfonic acid is prepared from the:
(A) hydrolysis of benzene
(B) neutralization of benzene
(C) sulfonation of benzene
(D) both A and B
Answer
519.9k+ views
Hint :The organosulfur compound benzenesulfonic acid (conjugate base benzenesulfonate) has the formula $C_6H_6O_3S$. It is the most basic aromatic sulfonic acid. It forms white waxy solids or deliquescent sheet crystals that are soluble in water, ethanol, and benzene but insoluble in nonpolar solvents like diethyl ether. It is often stored in the form of alkali metal salts. It has a very acidic aqueous solution.
Complete Step By Step Answer:
Benzenesulfonic acid is made by sulfonating benzene with concentrated sulfuric acid as follows:
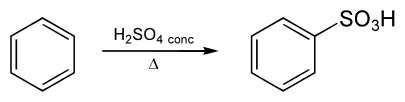
Sulfonation is a reversible reaction that produces benzenesulfonic acid by adding sulfur trioxide and fuming sulfuric acid. A concentrated solution of dissolved sulphur trioxide in sulfuric acid is known as fuming sulfuric acid, or oleum. Because oxygen is very electronegative, the oxygens in sulphur trioxide pull electrons away from it, making the sulphur electrophilic. To make benzenesulfonic acid, benzene attacks sulphur (and subsequent proton transfers occur). The reaction is reversed by adding hot aqueous acid to benzenesulfonic acid to produce benzene. Aromatic sulfonation, also known as "one of the most important reactions in industrial organic chemistry," is demonstrated in this conversion. Sulfonic acids are commonly used as a protecting group or as a meta director in electrophilic aromatic substitution.
When heated in water near $200 ^oC$ benzenesulfonic acid sulfonate. The temperature of sulfonation correlates with the ease of the sulfonation.
Hence, Option (C) is correct.
Note :
Surfactants in laundry detergent include benzenesulfonic acid salts such as Sodium benzenesulfonate (Ludigol) and Monoethanolamine benzenesulfonate. Besilates or besylates are benzene sulfonate salts that are used to make a variety of pharmaceutical drugs.
Complete Step By Step Answer:
Benzenesulfonic acid is made by sulfonating benzene with concentrated sulfuric acid as follows:

Sulfonation is a reversible reaction that produces benzenesulfonic acid by adding sulfur trioxide and fuming sulfuric acid. A concentrated solution of dissolved sulphur trioxide in sulfuric acid is known as fuming sulfuric acid, or oleum. Because oxygen is very electronegative, the oxygens in sulphur trioxide pull electrons away from it, making the sulphur electrophilic. To make benzenesulfonic acid, benzene attacks sulphur (and subsequent proton transfers occur). The reaction is reversed by adding hot aqueous acid to benzenesulfonic acid to produce benzene. Aromatic sulfonation, also known as "one of the most important reactions in industrial organic chemistry," is demonstrated in this conversion. Sulfonic acids are commonly used as a protecting group or as a meta director in electrophilic aromatic substitution.
When heated in water near $200 ^oC$ benzenesulfonic acid sulfonate. The temperature of sulfonation correlates with the ease of the sulfonation.
Hence, Option (C) is correct.
Note :
Surfactants in laundry detergent include benzenesulfonic acid salts such as Sodium benzenesulfonate (Ludigol) and Monoethanolamine benzenesulfonate. Besilates or besylates are benzene sulfonate salts that are used to make a variety of pharmaceutical drugs.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




