
How many banana bonds are there in diborane?
Answer
513k+ views
Hint: Diborane is the dimer of a borane molecule and has the chemical formula as ${B_2}{H_6}$ . In this compound, there are two types of bonds, one is normal covalent bond and the other one is banana bond. So, in order to know about how many banana bonds are present in diborane, we need to see its structure.
Complete answer:
Diborane is a dimer of borane molecules and it has the chemical formula as ${B_2}{H_6}$ . Diborane is an electrophile because according to octet rule, it requires fourteen electrons but it has only twelve electrons.
Now, if we look at the structure of diborane:
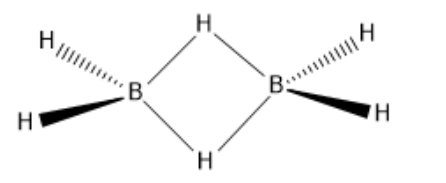
So, as we can see in this structure that there are four normal covalent bonds between boron and hydrogen (B-H) and the bond of B-H-B, it is the $3 - centre,2 - electron$ bond which means that there are three centres that is two borons and one hydrogen and only two electrons are present in this bond which are delocalised on these three centres.
So, we can say that the $3 - centre,2 - electron$ bond in B-H-B is known as the banana bond and there are a total two banana bonds which are present in the diborane.
Note:
As we have seen that there are two banana bonds in diborane, we know that these types of bonds are electron deficient and hence, this makes the diborane molecule electron deficient in nature. Therefore, it can be said that diborane is a strong electrophile and can accept lone pairs of electrons from a nucleophile to make covalent bonds.
Complete answer:
Diborane is a dimer of borane molecules and it has the chemical formula as ${B_2}{H_6}$ . Diborane is an electrophile because according to octet rule, it requires fourteen electrons but it has only twelve electrons.
Now, if we look at the structure of diborane:

So, as we can see in this structure that there are four normal covalent bonds between boron and hydrogen (B-H) and the bond of B-H-B, it is the $3 - centre,2 - electron$ bond which means that there are three centres that is two borons and one hydrogen and only two electrons are present in this bond which are delocalised on these three centres.
So, we can say that the $3 - centre,2 - electron$ bond in B-H-B is known as the banana bond and there are a total two banana bonds which are present in the diborane.
Note:
As we have seen that there are two banana bonds in diborane, we know that these types of bonds are electron deficient and hence, this makes the diborane molecule electron deficient in nature. Therefore, it can be said that diborane is a strong electrophile and can accept lone pairs of electrons from a nucleophile to make covalent bonds.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




