
How many atoms of nitrogen \[\left( N \right)\] are found in\[{\left( {N{H_4}} \right)_2}C{O_3}\]?
Answer
557.4k+ views
Hint: We need to remember that an atom is the smallest unit of matter that retains all chemical properties of a component. Atoms combine to form molecules which then interact to make solids, gases or liquids. Protons, electrons and neutrons are three basic particles of atoms. The center of the atom is called nucleus and it contains the protons and neutrons, electrons are present outside the nucleus of an atom.
Complete step by step answer:
We need to know that the atoms combine to make molecules. For instance, water $({H_2}O)$ consists of hydrogen oxygen atoms that have combined to make water molecules. Many biological processes are dedicated to breaking down molecules into their compound atoms in order that they often resemble a more useful molecule.
The given compound \[{\left( {N{H_4}} \right)_2}C{O_3}\] , chemical name is ammonium carbonate. It is an ionic compound made from ammonium cations, \[{(N{H_4})^ + }\] cations and carbonate ions, \[C{O_3}^{2 - }\].
\[{\left( {N{H_4}} \right)_2}C{O_3}\] contains four elements namely nitrogen \[\left( N \right)\], hydrogen $(H)$, carbon $(C)$ and oxygen .
Now it is vital to understand that so as for carbonate to be neutral, we’d like the general charge of the cation to balance the general charge of the anion.
In the ammonium nitrate the $2$(\[{\left( {N{H_4}} \right)_2}\]) subscripts apply to everything contained in the parenthesis.
And the positive charge of the ammonium cation tells you that, you simply need two such type cations so as to balance out the $2$ negative charge of the carbonate anion.
Hence, one ammonium cation contains,
$1 \times N$ , one nitrogen atom and four hydrogen atoms \[(4 \times H)\] .
And it follows that two ammonium cations will carry,
Two nitrogen atoms and eight hydrogen atoms.
Consequently, one formula unit of carbonate will contain two atoms of nitrogen$(2 \times N)$. And the structure of the ammonium carbonate is,
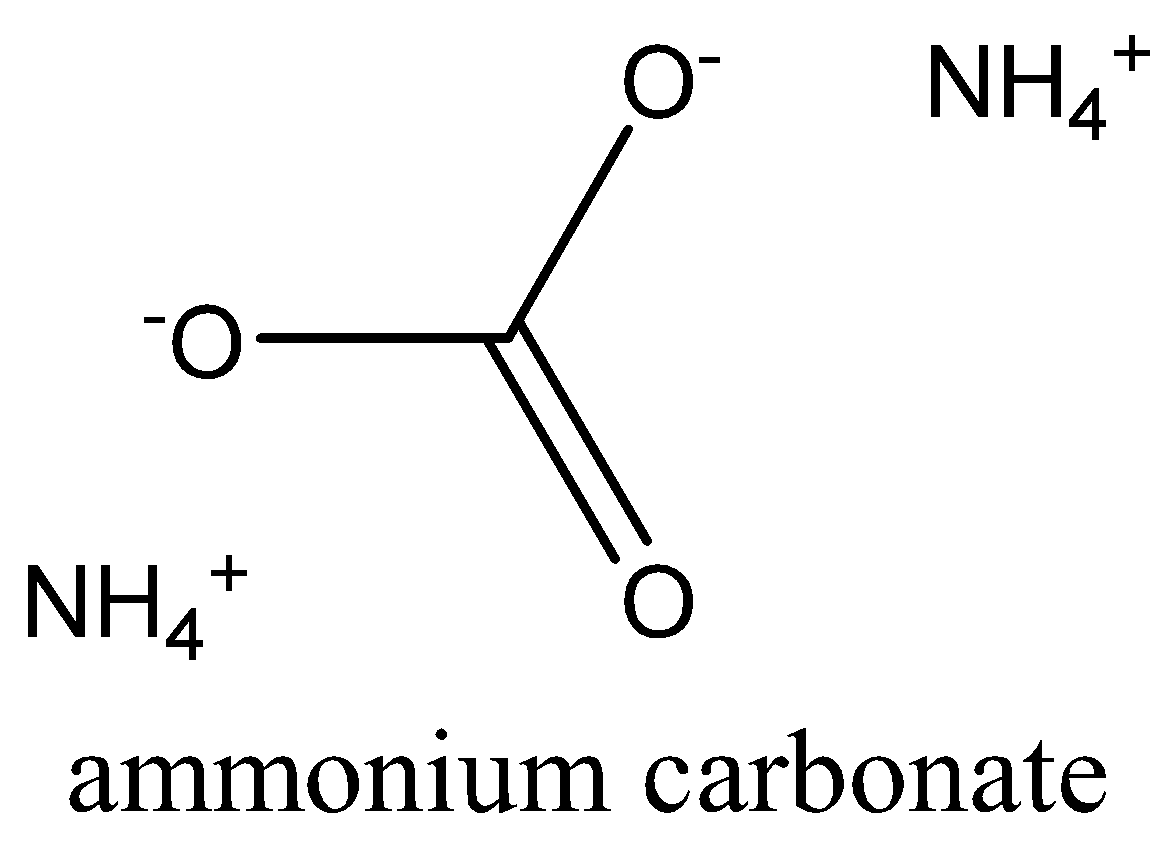
Note:
Do not confuse atom and element. An element part is called an atom. A specific element consists of just one sort of atom. Protons, electrons and neutrons are three basic particles (also subatomic particles) of atoms. Elements can combine with one another to make molecules via reaction.
Molecule: It is a substance and also two or more atoms bonded together. The simplest unit of a compound or an element. For example, ${O_2}$.
Compound: Two or more elements bonded together, like salt $(NaCl)$.
Complete step by step answer:
We need to know that the atoms combine to make molecules. For instance, water $({H_2}O)$ consists of hydrogen oxygen atoms that have combined to make water molecules. Many biological processes are dedicated to breaking down molecules into their compound atoms in order that they often resemble a more useful molecule.
The given compound \[{\left( {N{H_4}} \right)_2}C{O_3}\] , chemical name is ammonium carbonate. It is an ionic compound made from ammonium cations, \[{(N{H_4})^ + }\] cations and carbonate ions, \[C{O_3}^{2 - }\].
\[{\left( {N{H_4}} \right)_2}C{O_3}\] contains four elements namely nitrogen \[\left( N \right)\], hydrogen $(H)$, carbon $(C)$ and oxygen .
Now it is vital to understand that so as for carbonate to be neutral, we’d like the general charge of the cation to balance the general charge of the anion.
In the ammonium nitrate the $2$(\[{\left( {N{H_4}} \right)_2}\]) subscripts apply to everything contained in the parenthesis.
And the positive charge of the ammonium cation tells you that, you simply need two such type cations so as to balance out the $2$ negative charge of the carbonate anion.
Hence, one ammonium cation contains,
$1 \times N$ , one nitrogen atom and four hydrogen atoms \[(4 \times H)\] .
And it follows that two ammonium cations will carry,
Two nitrogen atoms and eight hydrogen atoms.
Consequently, one formula unit of carbonate will contain two atoms of nitrogen$(2 \times N)$. And the structure of the ammonium carbonate is,

Note:
Do not confuse atom and element. An element part is called an atom. A specific element consists of just one sort of atom. Protons, electrons and neutrons are three basic particles (also subatomic particles) of atoms. Elements can combine with one another to make molecules via reaction.
Molecule: It is a substance and also two or more atoms bonded together. The simplest unit of a compound or an element. For example, ${O_2}$.
Compound: Two or more elements bonded together, like salt $(NaCl)$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




