
Assertion:In Lewis structure of ${\text{N}}{{\text{F}}_{\text{3}}}{\text{ and C}}{{\text{O}}_{\text{3}}}^{{\text{2 - }}}$ nitrogen and carbon occupy the central position whereas fluorine and oxygen occupy the terminal positions.
Reason:
In Lewis representation, the least electronegative atom occupies the central position in the molecule/ion.
A) Both Assertion and Reason are correct, and Reason is the correct explanation for Assertion
B) Both Assertion and Reason are correct, but Reason is not the correct explanation for Assertion
C) Assertion is correct but Reason is incorrect
D) Both Assertion and Reason are incorrect
Answer
563.4k+ views
Hint: In Lewis structure, we will represent an atom by its chemical symbol. And then draw the atom’s valence electrons as dots around the atom. To draw the Lewis structure for a molecule just draw each atom with all its valence electrons. Unpaired electrons of two different atoms can come together to make covalent bonds. Now, instead of two dots we make a line which represents a covalent bond that always contains two electrons.
Complete step by step answer:
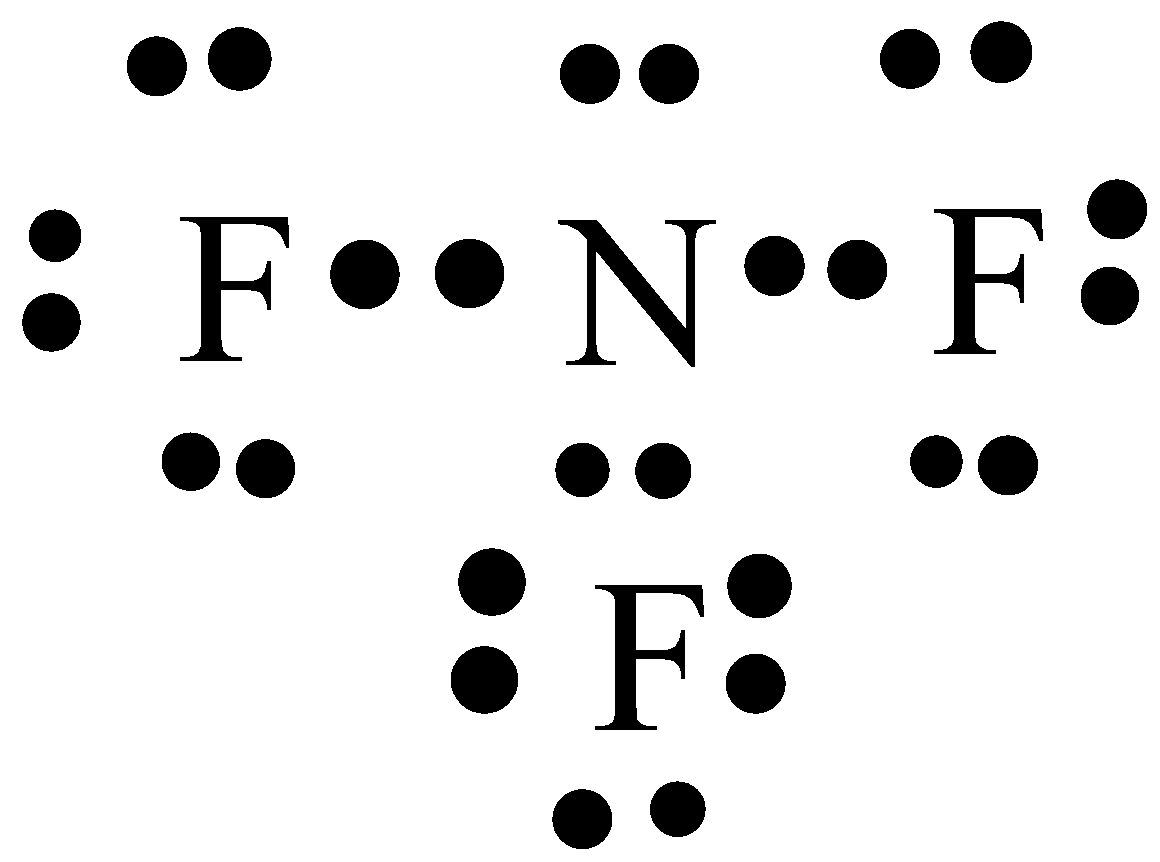
Let’s see the Lewis structure of nitrogen trifluoride ${\text{N}}{{\text{F}}_{\text{3}}}$:
Nitrogen is in group 5 or 15 on the periodic table. So, it has 5 valence electrons. Fluorine is in group 7 or 17. So, it has 7 valence electrons. So, ${\text{N}}{{\text{F}}_{\text{3}}}{\text{ has 5 + 7(3) = 26}}$ valence electrons. Nitrogen is the least electronegative. So, it is going to be in the centre surrounded by three fluorines. So, we will arrange these ${\text{26}}$ valence electrons in such a way that each element must get ${\text{8}}$ valence electrons or an octet.
Let’s see the Lewis structure of carbonate ion, ${\text{C}}{{\text{O}}_{\text{3}}}^{{\text{2 - }}}$. Carbon has ${\text{4}}$ valence electrons and Oxygen has ${\text{6}}$. And here we have ${\text{3}}$ oxygens and a negative ${\text{2}}$ i.e., we have an extra two valence electrons. Thus, we will have ${\text{4 + 6(3) + 2 = 24}}$ valence electrons all together. Carbon is least electronegative and hence it’ll be at the centre and three oxygens go around.
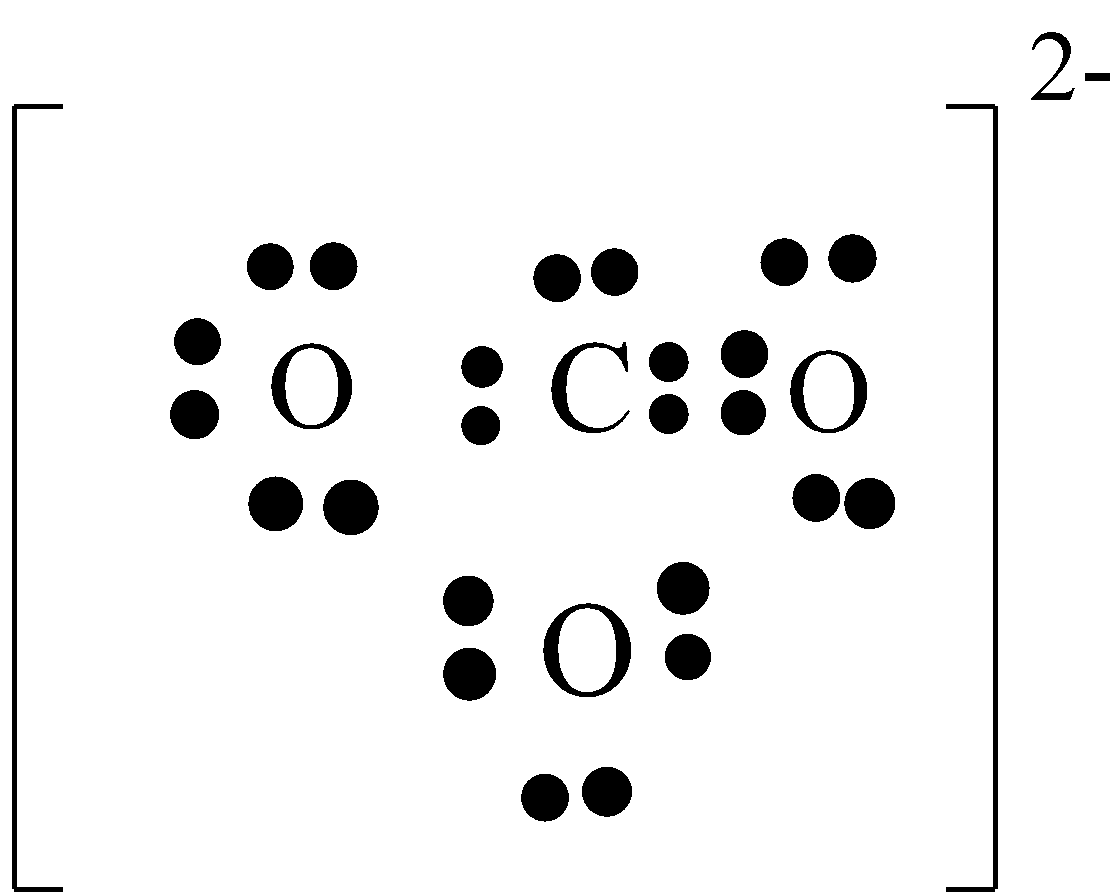
Here, the formal charges for the two oxygens is negative i.e., ${\text{ - 1 and - 1}}$ and the oxygen that is sharing its two valence electrons with carbon and the carbon has formal charge of ${\text{0}}$. And because the ${\text{C}}{{\text{O}}_{\text{3}}}^{{\text{2 - }}}$ has a charge of negative ${\text{2}}$, we need to put brackets around our Lewis structure and put a negative ${\text{2}}$ outside the bracket.
Hence, in Lewis representation, the least electronegative atom occupies the central position in the molecule/ion. Hydrogen and the halogens are usually terminal.
Hence, the answer is option A.
Additional information:
Lewis structure is based always
On the outermost electrons i.e., valence electrons and
On the Octet rule i.e., ${\text{8}}{{\text{e}}^{\text{ - }}}$ in its valence shell except for hydrogen
Octet rule can be neglected for the central atom
Note: The rule for selecting central atom: which is at least number of atoms or which has least electronegativity or which has largest size, or which has highest atomic number. Hydrogen and fluorine can never be the central atom.
Complete step by step answer:
Let’s see the Lewis structure of nitrogen trifluoride ${\text{N}}{{\text{F}}_{\text{3}}}$:
Nitrogen is in group 5 or 15 on the periodic table. So, it has 5 valence electrons. Fluorine is in group 7 or 17. So, it has 7 valence electrons. So, ${\text{N}}{{\text{F}}_{\text{3}}}{\text{ has 5 + 7(3) = 26}}$ valence electrons. Nitrogen is the least electronegative. So, it is going to be in the centre surrounded by three fluorines. So, we will arrange these ${\text{26}}$ valence electrons in such a way that each element must get ${\text{8}}$ valence electrons or an octet.

Let’s see the Lewis structure of carbonate ion, ${\text{C}}{{\text{O}}_{\text{3}}}^{{\text{2 - }}}$. Carbon has ${\text{4}}$ valence electrons and Oxygen has ${\text{6}}$. And here we have ${\text{3}}$ oxygens and a negative ${\text{2}}$ i.e., we have an extra two valence electrons. Thus, we will have ${\text{4 + 6(3) + 2 = 24}}$ valence electrons all together. Carbon is least electronegative and hence it’ll be at the centre and three oxygens go around.

Here, the formal charges for the two oxygens is negative i.e., ${\text{ - 1 and - 1}}$ and the oxygen that is sharing its two valence electrons with carbon and the carbon has formal charge of ${\text{0}}$. And because the ${\text{C}}{{\text{O}}_{\text{3}}}^{{\text{2 - }}}$ has a charge of negative ${\text{2}}$, we need to put brackets around our Lewis structure and put a negative ${\text{2}}$ outside the bracket.
Hence, in Lewis representation, the least electronegative atom occupies the central position in the molecule/ion. Hydrogen and the halogens are usually terminal.
Hence, the answer is option A.
Additional information:
Lewis structure is based always
On the outermost electrons i.e., valence electrons and
On the Octet rule i.e., ${\text{8}}{{\text{e}}^{\text{ - }}}$ in its valence shell except for hydrogen
Octet rule can be neglected for the central atom
Note: The rule for selecting central atom: which is at least number of atoms or which has least electronegativity or which has largest size, or which has highest atomic number. Hydrogen and fluorine can never be the central atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




