
ASSERTION
The second law of thermodynamics states that the entropy of a closed or isolated system always increases. This means that all available energy is used up and there is no more potential for further useful work.
REASON
The system becomes disordered and also degraded.
A) Both Assertion and Reason are correct and Reason is the correct explanation for Assertion
B) Both Assertion and Reason are correct but Reason is not correct explanation for Assertion
C) Assertion is correct but reason is in correct
D) Both Assertion and Reason is incorrect
Answer
529.2k+ views
Hint:In this statement above is a measure of the system's randomness or instability within an isolated system. It can be regarded as a quantitative index that describes energy quality. For any random operation, we determine the correct statement that will always lead to an escalation in the entropy of the universe.
Useful formula:
Second law of thermodynamics formula
$\Delta S = Entropy = \dfrac{{\Delta Q}}{T}$
Where,
$\Delta S$ is Entropy
$\Delta Q$ is the heat transfer to or from the system.
$T$ is the absolute temperature at the boundary.
${S_r} = {S_i}$
Reversible
${S_r} > {S_{_i}}$
Irreversible
Where, ${S_r}$ reversible ${S_i}$ irreversible
Complete step by step procedure:
Given by,
Assertion and Reason
We find the correct statement.
Degraded energy is energy that becomes unavailable which means it is no longer able to produce work.
Degraded energy has a connection with the second law of thermodynamics.
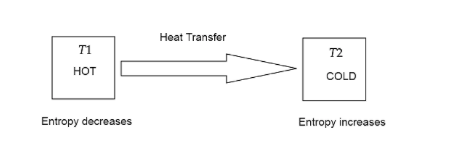
The second law of thermodynamics states that the entropy of a closed or isolated system always increases.
$\Delta S = Entropy = \dfrac{{\Delta Q}}{T}$
${S_r} = {S_i}$
Reversible
${S_r} > {S_{_i}}$
Irreversible
This means that all available energy is used up and there is no more potential for further useful work. The system becomes disordered and also degraded. This is also called the Entropy Law.
Hence, option A is the correct answer.
Both Assertion and Reason are correct and Reason is the correct explanation for Assertion.
Note:Although the mass remains constant, there is a heat exchange with the surroundings in a closed system. This change in the heat content disrupts the system, thereby increasing the system's entropy. Internal changes can occur in the movements of the system's molecules. This leads to disruptions that further cause the system to become irreversible, leading to an increase in its entropy.
Useful formula:
Second law of thermodynamics formula
$\Delta S = Entropy = \dfrac{{\Delta Q}}{T}$
Where,
$\Delta S$ is Entropy
$\Delta Q$ is the heat transfer to or from the system.
$T$ is the absolute temperature at the boundary.
${S_r} = {S_i}$
Reversible
${S_r} > {S_{_i}}$
Irreversible
Where, ${S_r}$ reversible ${S_i}$ irreversible
Complete step by step procedure:
Given by,
Assertion and Reason
We find the correct statement.
Degraded energy is energy that becomes unavailable which means it is no longer able to produce work.
Degraded energy has a connection with the second law of thermodynamics.

The second law of thermodynamics states that the entropy of a closed or isolated system always increases.
$\Delta S = Entropy = \dfrac{{\Delta Q}}{T}$
${S_r} = {S_i}$
Reversible
${S_r} > {S_{_i}}$
Irreversible
This means that all available energy is used up and there is no more potential for further useful work. The system becomes disordered and also degraded. This is also called the Entropy Law.
Hence, option A is the correct answer.
Both Assertion and Reason are correct and Reason is the correct explanation for Assertion.
Note:Although the mass remains constant, there is a heat exchange with the surroundings in a closed system. This change in the heat content disrupts the system, thereby increasing the system's entropy. Internal changes can occur in the movements of the system's molecules. This leads to disruptions that further cause the system to become irreversible, leading to an increase in its entropy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




