
Arrhenius equation is $k=A{{e}^{\dfrac{-{{E}_{a}}}{RT}}}$. Which of the following graphs represents the variation of rate constant k against the temperature T?
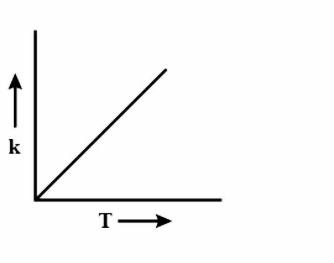
(A)
(B)
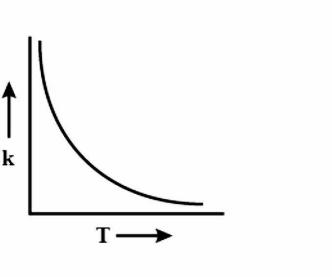
(C)
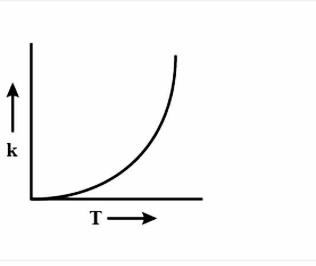
(D)
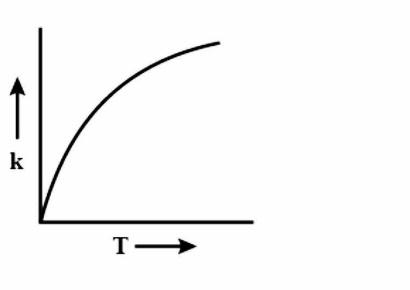




Answer
590.1k+ views
Note: You must recall the fact that when the temperature is increased in a chemical reaction, then the rate constant also increases. For every \[{{10}^{0}}\] rise in temperature, the rate constant gets nearly doubled.
Complete Step-by-step solution:
First, let us recall the Arrhenius Equation for chemical kinetics. The Arrhenius equation is represented as:
$k=A{{e}^{\dfrac{-{{E}_{a}}}{RT}}}$ , k is the rate constant,
A is the Arrhenius factor,
${{E}_{a}}$ is the activation energy.
During every chemical reaction, an intermediate is formed, in the beginning. In order for the intermediate to form, some energy is required and this energy is called the Activation energy. A lot of collisions happen between the molecules during the reaction and these collisions are responsible for formation of the products. Now, if the temperature is increased, then heat is transferred in the form of Kinetic energy. The fraction of molecules which collide increases, which have energy more than the activation energy \[{{E}_{a}}\]. As the graph is increasing, so accordingly the options are eliminated. Now to figure out the shape of the graph, we have to again look at the equation i.e $k=A{{e}^{\dfrac{-{{E}_{a}}}{RT}}}$. Clearly, the equation is an exponential graph so it will be curved and not be in a straight line. Hence, it is either B or D. The last thing we can look at is the sign of the exponent.
We can observe that it is a negative exponential power graph, so in that case, our answer will be option C, i.e negative exponential.
Note: No chemical reaction can occur if the fraction of molecules have energies less than the activation energy \[{{E}_{a}}\]. Moreover, you can put random values of k and \[{{E}_{a}}\], A in the equation and check which graph satisfies it, in case time is less during the exam.
Complete Step-by-step solution:
First, let us recall the Arrhenius Equation for chemical kinetics. The Arrhenius equation is represented as:
$k=A{{e}^{\dfrac{-{{E}_{a}}}{RT}}}$ , k is the rate constant,
A is the Arrhenius factor,
${{E}_{a}}$ is the activation energy.
During every chemical reaction, an intermediate is formed, in the beginning. In order for the intermediate to form, some energy is required and this energy is called the Activation energy. A lot of collisions happen between the molecules during the reaction and these collisions are responsible for formation of the products. Now, if the temperature is increased, then heat is transferred in the form of Kinetic energy. The fraction of molecules which collide increases, which have energy more than the activation energy \[{{E}_{a}}\]. As the graph is increasing, so accordingly the options are eliminated. Now to figure out the shape of the graph, we have to again look at the equation i.e $k=A{{e}^{\dfrac{-{{E}_{a}}}{RT}}}$. Clearly, the equation is an exponential graph so it will be curved and not be in a straight line. Hence, it is either B or D. The last thing we can look at is the sign of the exponent.
We can observe that it is a negative exponential power graph, so in that case, our answer will be option C, i.e negative exponential.
Note: No chemical reaction can occur if the fraction of molecules have energies less than the activation energy \[{{E}_{a}}\]. Moreover, you can put random values of k and \[{{E}_{a}}\], A in the equation and check which graph satisfies it, in case time is less during the exam.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Give simple chemical tests to distinguish between the class 12 chemistry CBSE

Define Vant Hoff factor How is it related to the degree class 12 chemistry CBSE




