
Arrange the following molecules in the decreasing order of their dipole moment:
A. $ CB{{r}_{4}} $
B. $ CHB{{r}_{3}} $
C. $ C{{H}_{2}}B{{r}_{2}} $
D. $ C{{H}_{3}}Br $
Answer
561k+ views
Hint As the number of hydrogens increase, the polarity also increases. The compounds which have symmetrical shape have 0 or less dipole moment as opposite dipole moment vectors cancel out each other.
Complete step by step solution:
In order to answer our question, we need to learn about the dipole moment. Now, dipole moment is used to measure the polarity of a compound. When there are positive and negative charges present at a distance, then dipole moment occurs. Dipole moment is dependent upon the nature of charges, the magnitude of charges as well as the distance between both the charges. The dipole moment is represented by the symbol $ \mu $ . Mathematically, we can write:
\[\mu =q\times d\]
Where $ \mu $ is the dipole moment, q is the magnitude of the charged particle and d is the distance between the charges. The value of the dipole moment is always a vector quantity. More the dipole moment of a compound, more is its polarity. And more the polarity of the compound, the more stable it is. Now, let us come to the question and study the compounds. We can see that the number of bromines is gradually decreasing and hydrogen is occupying its space. So, let us analyse the dipole moment by looking at the structures of the 4 molecules:
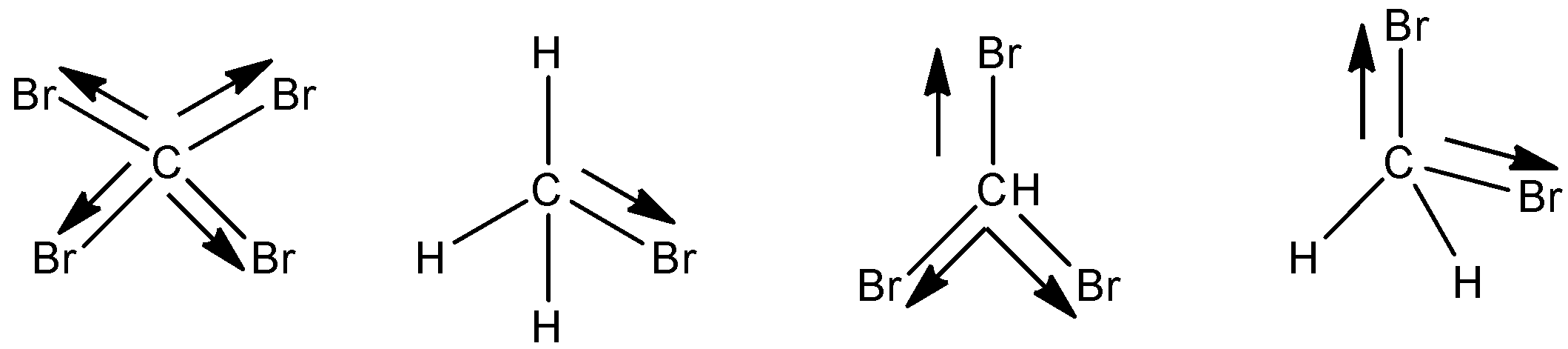
So, we can say that in $ CB{{r}_{4}} $ the dipole moment becomes 0 due to its symmetrical figure, as the dipoles cancel out each other. Hence it has the lowest dipole. The dipole of $ C{{H}_{2}}B{{r}_{2}} $ has more dipole moment than it as two bromines carry negative charge. Then as the hydrogens increase, the polarity increases. So, after that, $ CHB{{r}_{3}} $ , and followed by $ C{{H}_{3}}Br $ , has the highest dipole moment among the four.
NOTE: Dipole moment can be also considered similar to electric dipole and since it is a vector, it follows the vector law of addition too.
Complete step by step solution:
In order to answer our question, we need to learn about the dipole moment. Now, dipole moment is used to measure the polarity of a compound. When there are positive and negative charges present at a distance, then dipole moment occurs. Dipole moment is dependent upon the nature of charges, the magnitude of charges as well as the distance between both the charges. The dipole moment is represented by the symbol $ \mu $ . Mathematically, we can write:
\[\mu =q\times d\]
Where $ \mu $ is the dipole moment, q is the magnitude of the charged particle and d is the distance between the charges. The value of the dipole moment is always a vector quantity. More the dipole moment of a compound, more is its polarity. And more the polarity of the compound, the more stable it is. Now, let us come to the question and study the compounds. We can see that the number of bromines is gradually decreasing and hydrogen is occupying its space. So, let us analyse the dipole moment by looking at the structures of the 4 molecules:

So, we can say that in $ CB{{r}_{4}} $ the dipole moment becomes 0 due to its symmetrical figure, as the dipoles cancel out each other. Hence it has the lowest dipole. The dipole of $ C{{H}_{2}}B{{r}_{2}} $ has more dipole moment than it as two bromines carry negative charge. Then as the hydrogens increase, the polarity increases. So, after that, $ CHB{{r}_{3}} $ , and followed by $ C{{H}_{3}}Br $ , has the highest dipole moment among the four.
NOTE: Dipole moment can be also considered similar to electric dipole and since it is a vector, it follows the vector law of addition too.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




