
Arrange the following carbonyl compound in increasing order of their nucleophilic addition reaction.
Benzaldehyde, p-tolualdehyde, p-nitrobenzaldehyde, acetophenone.
Answer
555k+ views
Hint: Nucleophilic addition reaction is an additional reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile such that the double or triple bond is broken. The group which increases electrophilicity of carbonyl carbon has greater reactivity towards nucleophilic addition reaction.
Complete step by step answer:
If an electron withdrawing group is attached to carbonyl carbon that increases the reactivity of that carbonyl compound towards nucleophilic addition reaction. While the electron donating group decreases electrophilicity of carbonyl carbon hence decreases reactivity.
\[{{ + I}}\] Effect (electron donating inductive effect) is more in ketone than in aldehyde. Hence ketone is less reactive towards nucleophilic addition reactions.
Let us see the structure of each compound given in the question
Benzaldehyde
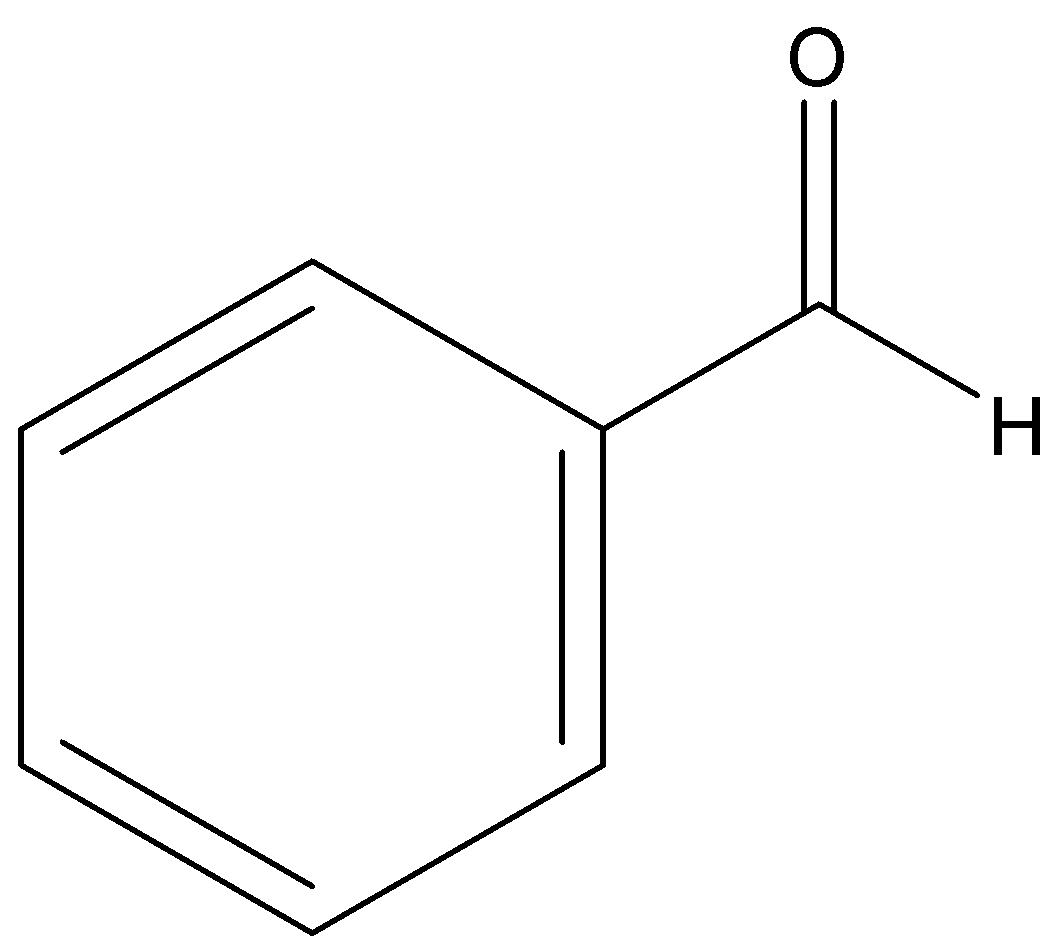
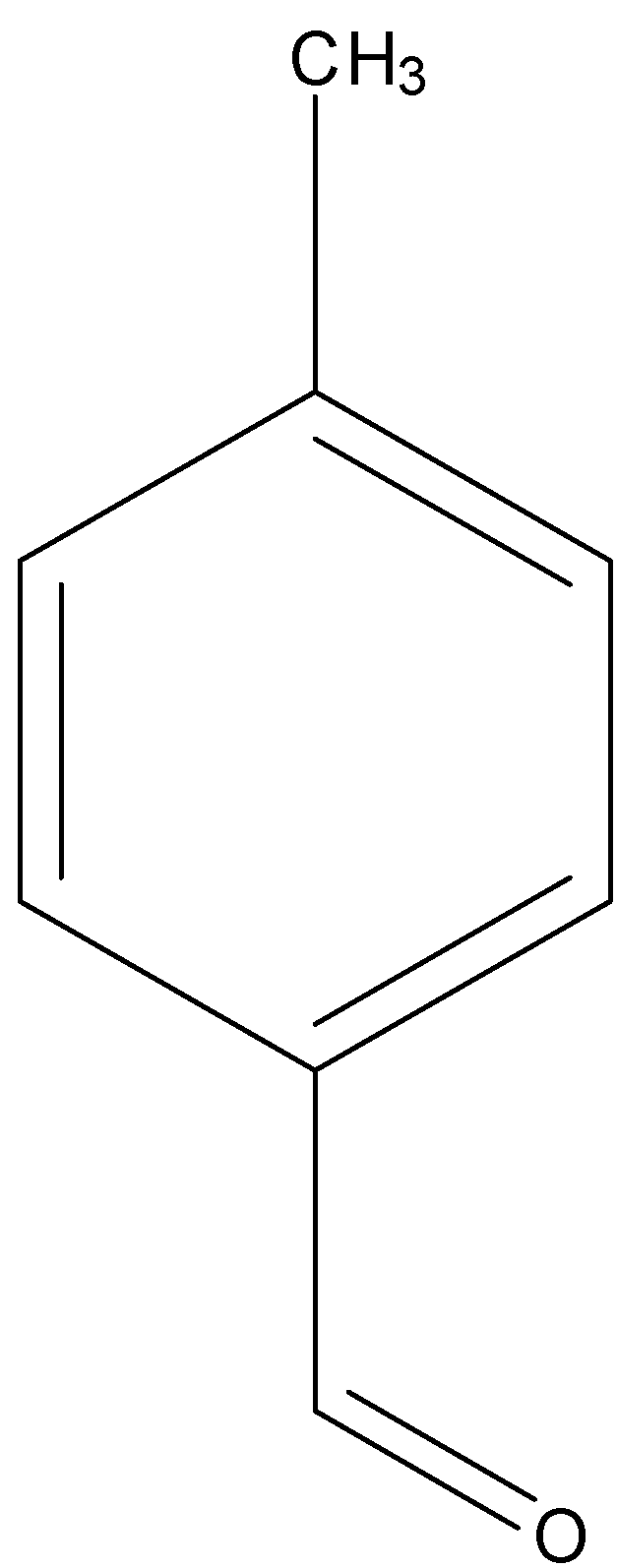
p-tolualdehyde
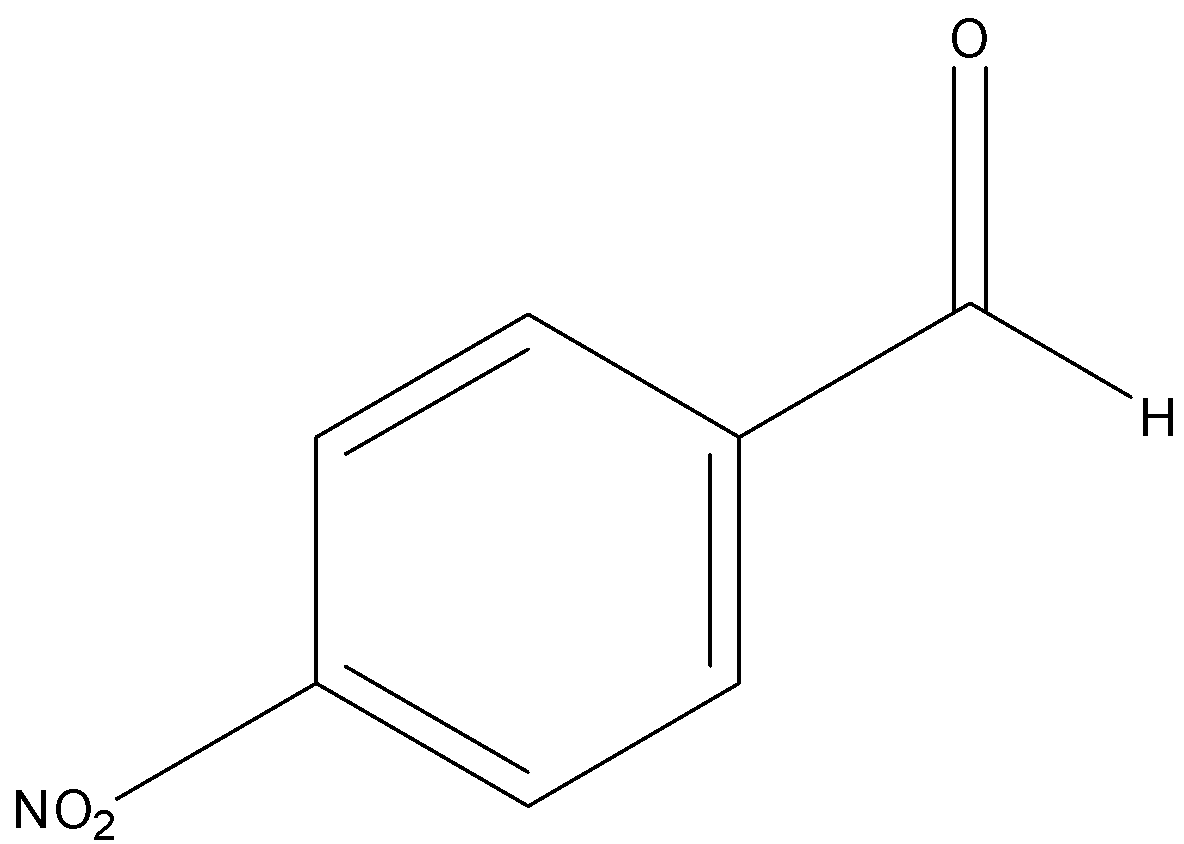
p-nitrobenzaldehyde
Acetophenone
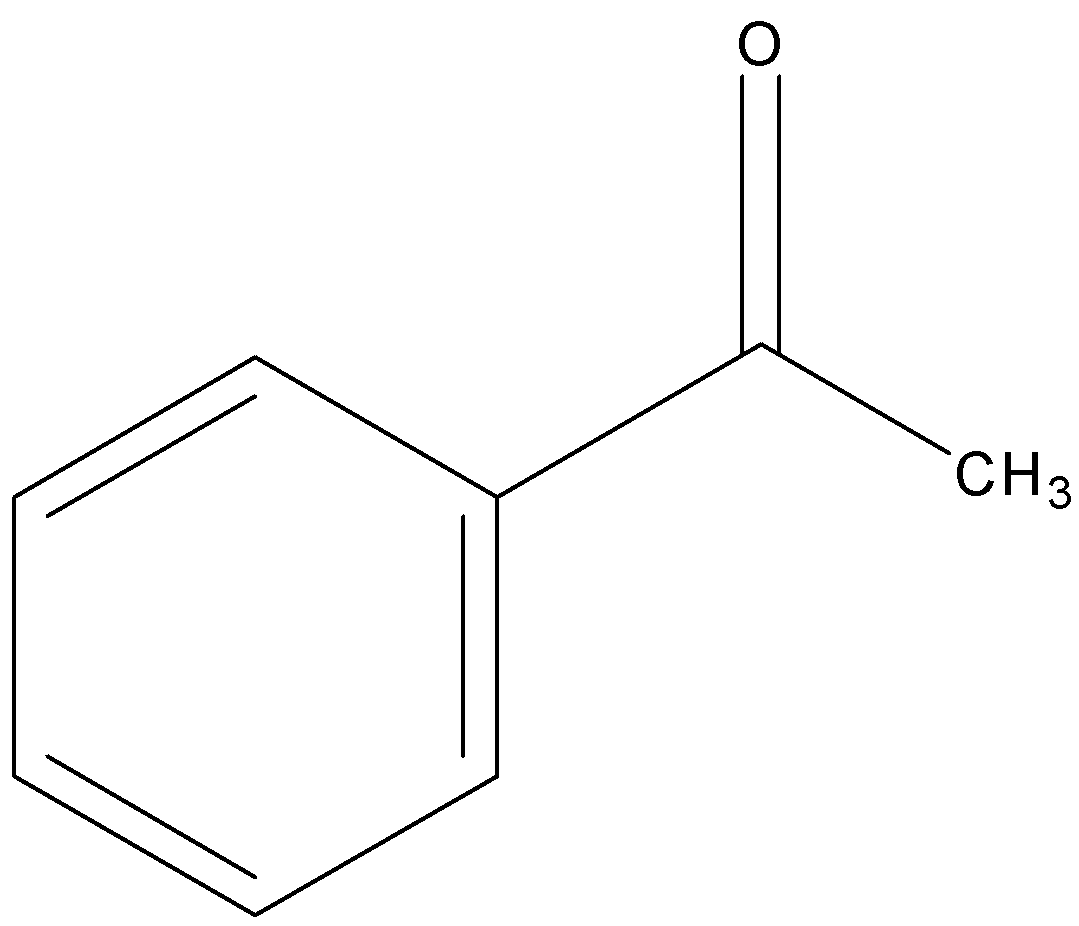
Hence out of Acetophenone (contains ketone functional group) is less reactive towards nucleophilic substitution reaction.
Among aldehydes, \[{{ + I}}\] effect is more in p-tolualdehyde due to electron donating \[\;C{H_3}\] group attached at para position to the carbonyl group. And lowest in p-nitrobenzaldehyde due to electron withdrawing \[\;N{O_2}\] group.
Hence order of their reactivity towards nucleophilic addition reaction is p-nitrobenzaldehyde, Benzaldehyde, p-tolualdehyde, acetophenone.
Additional information:
There are different types of nucleophilic addition reaction
For example
1) When aldehyde and ketone undergo nucleophilic addition reaction with \[HCN\] it produces cyanohydrin.
2) When aldehyde undergoes reaction with monohydric alcohols it forms hemiacetal.
Note: The nucleophilic addition reaction is initiated by addition of proton to the $\pi $ bond of the carbonyl group, forming a resonance stabilized carbocation. A molecule of alcohol bonds to the carbonyl carbon of this intermediate and forming a protonated alcohol.
Complete step by step answer:
If an electron withdrawing group is attached to carbonyl carbon that increases the reactivity of that carbonyl compound towards nucleophilic addition reaction. While the electron donating group decreases electrophilicity of carbonyl carbon hence decreases reactivity.
\[{{ + I}}\] Effect (electron donating inductive effect) is more in ketone than in aldehyde. Hence ketone is less reactive towards nucleophilic addition reactions.
Let us see the structure of each compound given in the question
Benzaldehyde

p-tolualdehyde

p-nitrobenzaldehyde

Acetophenone

Hence out of Acetophenone (contains ketone functional group) is less reactive towards nucleophilic substitution reaction.
Among aldehydes, \[{{ + I}}\] effect is more in p-tolualdehyde due to electron donating \[\;C{H_3}\] group attached at para position to the carbonyl group. And lowest in p-nitrobenzaldehyde due to electron withdrawing \[\;N{O_2}\] group.
Hence order of their reactivity towards nucleophilic addition reaction is p-nitrobenzaldehyde, Benzaldehyde, p-tolualdehyde, acetophenone.
Additional information:
There are different types of nucleophilic addition reaction
For example
1) When aldehyde and ketone undergo nucleophilic addition reaction with \[HCN\] it produces cyanohydrin.
2) When aldehyde undergoes reaction with monohydric alcohols it forms hemiacetal.
Note: The nucleophilic addition reaction is initiated by addition of proton to the $\pi $ bond of the carbonyl group, forming a resonance stabilized carbocation. A molecule of alcohol bonds to the carbonyl carbon of this intermediate and forming a protonated alcohol.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




