
Why are unsaturated hydrocarbons more reactive than saturated hydrocarbons? Give one example with an equation
Answer
535.5k+ views
Hint: Hydrocarbons are organic compounds which are formed due to the bonding between hydrogen atoms and carbon atoms. Saturated hydrocarbons have single bonded carbon atoms and hydrogen atoms fully occupying other positions whereas unsaturated hydrocarbons are compounds where carbon atoms have double or triple bonds which are weaker and can break down easily.
Complete step-by-step answer:Hydrocarbons are organic compounds and as the name suggests, they are formed due to the bonding between hydrogen atoms and carbons atoms. They exist as alkanes, alkenes, alkynes and aromatic compounds. Once the carbon atoms are bonded to each other, the vacant carbon bonds are filled with hydrogen atoms which take up all the positions which are available. Hydrocarbons are naturally occurring compounds which are used as fuels, lubricants and in production of plastics, rubber, solvents and various other products.
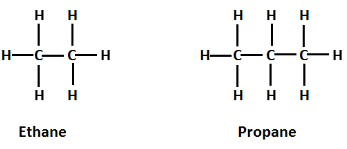
Saturated hydrocarbons are those in which carbon atoms are bonded to each other with a single bond and all the other bonds of the carbon atom are occupied by hydrogen atom. Therefore, these compounds are known as saturated as all the bonds of carbon after being single bonded with other carbon atoms are occupied by hydrogen atoms. When these saturated hydrocarbons have no functional groups attached then they are known as alkanes.
Some examples of saturated hydrocarbons are:
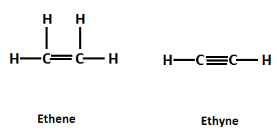
Unsaturated hydrocarbons are those hydrocarbons in which carbon atoms are covalently bonded to other carbon atoms by a double or a triple bond which reduces the number of hydrogen atoms attached to each carbon atom. They are formed due to the sharing of electrons. Double bonded hydrocarbons are known as alkenes and triple bonded are known as alkynes.
Some examples of unsaturated hydrocarbons are:
The unsaturated hydrocarbons are more reactive due to the presence of double and triple bonded carbon atoms as these are weaker than the single bonded saturated hydrocarbons due to the presence of weaker pi bonds and thus, when a reaction takes place, these unsaturated hydrocarbons break down easily as compared to single bonded carbon compounds and react faster when used in a reaction.
Unsaturated hydrocarbons easily undergo additional reactions like bromination whereas saturated hydrocarbons do not react with bromine due to single bonds.
$C{H_3}C{H_2}C{H_3} + Br \to \text{no reaction}$
Saturated hydrocarbon $\to$ Unsaturated hydrocarbon
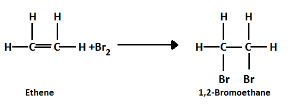
Note: Unsaturated hydrocarbons are used for various industrial purposes like production of paints, lubricants and pesticides. They are reactive and therefore used in various chemical reactions. They are healthier for our body than saturated hydrocarbons as they reduce bad cholesterol and prevent heart diseases.
Complete step-by-step answer:Hydrocarbons are organic compounds and as the name suggests, they are formed due to the bonding between hydrogen atoms and carbons atoms. They exist as alkanes, alkenes, alkynes and aromatic compounds. Once the carbon atoms are bonded to each other, the vacant carbon bonds are filled with hydrogen atoms which take up all the positions which are available. Hydrocarbons are naturally occurring compounds which are used as fuels, lubricants and in production of plastics, rubber, solvents and various other products.
Saturated hydrocarbons are those in which carbon atoms are bonded to each other with a single bond and all the other bonds of the carbon atom are occupied by hydrogen atom. Therefore, these compounds are known as saturated as all the bonds of carbon after being single bonded with other carbon atoms are occupied by hydrogen atoms. When these saturated hydrocarbons have no functional groups attached then they are known as alkanes.
Some examples of saturated hydrocarbons are:

Unsaturated hydrocarbons are those hydrocarbons in which carbon atoms are covalently bonded to other carbon atoms by a double or a triple bond which reduces the number of hydrogen atoms attached to each carbon atom. They are formed due to the sharing of electrons. Double bonded hydrocarbons are known as alkenes and triple bonded are known as alkynes.
Some examples of unsaturated hydrocarbons are:

The unsaturated hydrocarbons are more reactive due to the presence of double and triple bonded carbon atoms as these are weaker than the single bonded saturated hydrocarbons due to the presence of weaker pi bonds and thus, when a reaction takes place, these unsaturated hydrocarbons break down easily as compared to single bonded carbon compounds and react faster when used in a reaction.
Unsaturated hydrocarbons easily undergo additional reactions like bromination whereas saturated hydrocarbons do not react with bromine due to single bonds.
$C{H_3}C{H_2}C{H_3} + Br \to \text{no reaction}$
Saturated hydrocarbon $\to$ Unsaturated hydrocarbon

Note: Unsaturated hydrocarbons are used for various industrial purposes like production of paints, lubricants and pesticides. They are reactive and therefore used in various chemical reactions. They are healthier for our body than saturated hydrocarbons as they reduce bad cholesterol and prevent heart diseases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




