
What are the three geometric isomers of dibenzalacetone?
Answer
535.2k+ views
Hint: The IUPAC name of dibenzalacetone is 1,5-Diphenylpenta-1,4-dien-3-one. It is a saturated hydrocarbon having double bonds. The compounds having double bonds show cis and trans isomerism due to different orientations of groups around the double bond.
Complete answer:
Dibenzalacetone is a saturated organic compound with the chemical formula: ${{\text{C}}_{6}}{{\text{H}}_{\text{5}}}\text{CH=CHCOCH=CH}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}$. It is a pale-yellow solid and is used in the making of sunscreens.
It is synthesized by aldol condensation of acetone with benzaldehyde. The first step involves the reaction of one mole of acetone and one mole of benzaldehyde to form benzylideneacetone.
\[\begin{align}
& {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{CHO}+\text{C}{{\text{H}}_{\text{3}}}\text{COC}{{\text{H}}_{\text{3}}}\xrightarrow{\text{NaOH}}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{CH=CHCOC}{{\text{H}}_{\text{3}}}+{{\text{H}}_{\text{2}}}\text{O} \\
& \text{(Benzaldehyde)}+(\text{Acetone)}\xrightarrow{\text{NaOH}}\text{(Benzylidineacetone)}+(\text{Water)} \\
\end{align}\]
This ketone formed then further undergoes aldol condensation with the second mole of benzaldehyde and produces dibenzalacetone.
\[\begin{align}
& {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{CHO}+{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{CH=CHCOC}{{\text{H}}_{\text{3}}}\xrightarrow{\text{NaOH}}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{CH=CHCOCH=CH}{{\text{C}}_{6}}{{\text{H}}_{5}} \\
& \text{(Benzaldehyde)}+\text{(Benzylidineacetone)}\xrightarrow{\text{NaOH}}\text{(Dibenzalacetone)} \\
\end{align}\]
This dibenzalacetone can show cis-trans geometrical isomerism due to the presence of a double bond. The possible isomeric structures are cis-trans, cis-cis, trans-trans, and trans-cis as shown below.
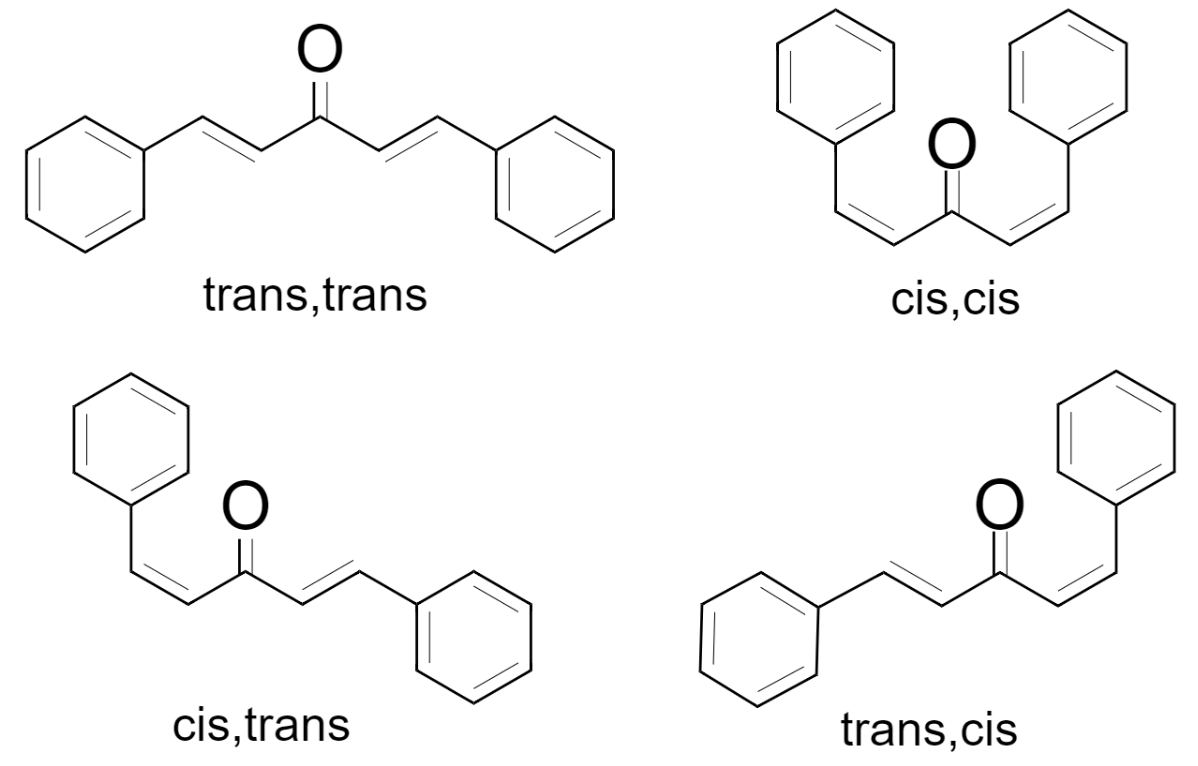
Out of all these, the cis-trans and trans-cis isomers are identical and thus they are considered as only one geometrical isomer of dibenzalacetone.
Thus, there are a total of three possible geometrical isomers of dibenzalacetone. They are –
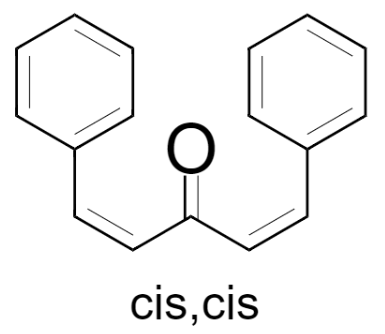
(1) cis, cis – 1,5-Diphenylpenta-1,4-dien-3-one
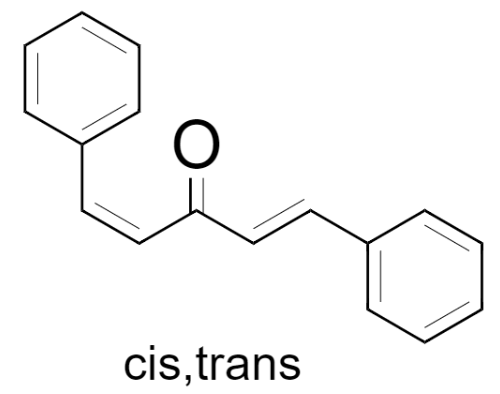
(2) cis, trans – 1,5-Diphenylpenta-1,4-dien-3-one
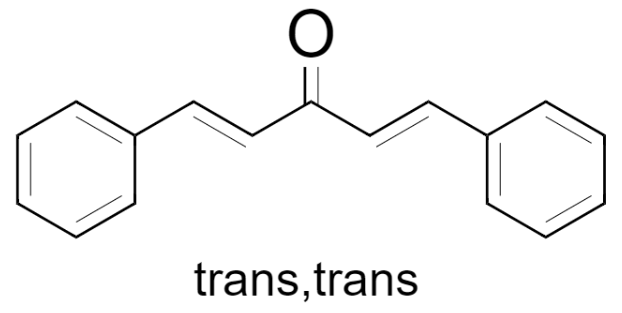
(3) trans, trans – 1,5-Diphenylpenta-1,4-dien-3-one
Note:
Aldol condensation is a reaction in which two aldehydes, or two ketones, or one aldehyde and one ketone react in presence of a dilute base to form $\beta $-hydroxy ketone or $\beta $-hydroxy aldehyde that after dehydration yields a conjugated enone. The above reaction of dibenzalacetone formation is an example of an aldol condensation.
Complete answer:
Dibenzalacetone is a saturated organic compound with the chemical formula: ${{\text{C}}_{6}}{{\text{H}}_{\text{5}}}\text{CH=CHCOCH=CH}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}$. It is a pale-yellow solid and is used in the making of sunscreens.
It is synthesized by aldol condensation of acetone with benzaldehyde. The first step involves the reaction of one mole of acetone and one mole of benzaldehyde to form benzylideneacetone.
\[\begin{align}
& {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{CHO}+\text{C}{{\text{H}}_{\text{3}}}\text{COC}{{\text{H}}_{\text{3}}}\xrightarrow{\text{NaOH}}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{CH=CHCOC}{{\text{H}}_{\text{3}}}+{{\text{H}}_{\text{2}}}\text{O} \\
& \text{(Benzaldehyde)}+(\text{Acetone)}\xrightarrow{\text{NaOH}}\text{(Benzylidineacetone)}+(\text{Water)} \\
\end{align}\]
This ketone formed then further undergoes aldol condensation with the second mole of benzaldehyde and produces dibenzalacetone.
\[\begin{align}
& {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{CHO}+{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{CH=CHCOC}{{\text{H}}_{\text{3}}}\xrightarrow{\text{NaOH}}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{CH=CHCOCH=CH}{{\text{C}}_{6}}{{\text{H}}_{5}} \\
& \text{(Benzaldehyde)}+\text{(Benzylidineacetone)}\xrightarrow{\text{NaOH}}\text{(Dibenzalacetone)} \\
\end{align}\]
This dibenzalacetone can show cis-trans geometrical isomerism due to the presence of a double bond. The possible isomeric structures are cis-trans, cis-cis, trans-trans, and trans-cis as shown below.

Out of all these, the cis-trans and trans-cis isomers are identical and thus they are considered as only one geometrical isomer of dibenzalacetone.
Thus, there are a total of three possible geometrical isomers of dibenzalacetone. They are –
(1) cis, cis – 1,5-Diphenylpenta-1,4-dien-3-one

(2) cis, trans – 1,5-Diphenylpenta-1,4-dien-3-one

(3) trans, trans – 1,5-Diphenylpenta-1,4-dien-3-one

Note:
Aldol condensation is a reaction in which two aldehydes, or two ketones, or one aldehyde and one ketone react in presence of a dilute base to form $\beta $-hydroxy ketone or $\beta $-hydroxy aldehyde that after dehydration yields a conjugated enone. The above reaction of dibenzalacetone formation is an example of an aldol condensation.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

Which out of the following hydrocarbons undergo addition class 11 chemistry CBSE




