
What are the products of hydrolysis of maltose?
Answer
592.2k+ views
Hint: Hydrolysis means using water molecules to break a chemical bond in a reaction. We can say that hydrolysis is the opposite reaction of condensation reaction where elimination of water happens in the bond formation. Hydrolysis of ester bonds forms an acid and an alcohol as the products.
Complete answer:
Maltose is a disaccharide consisting of two molecules of glucose with \[\alpha \]-(1,4) glycosidic linkage.
The structure of the maltose is as follows.
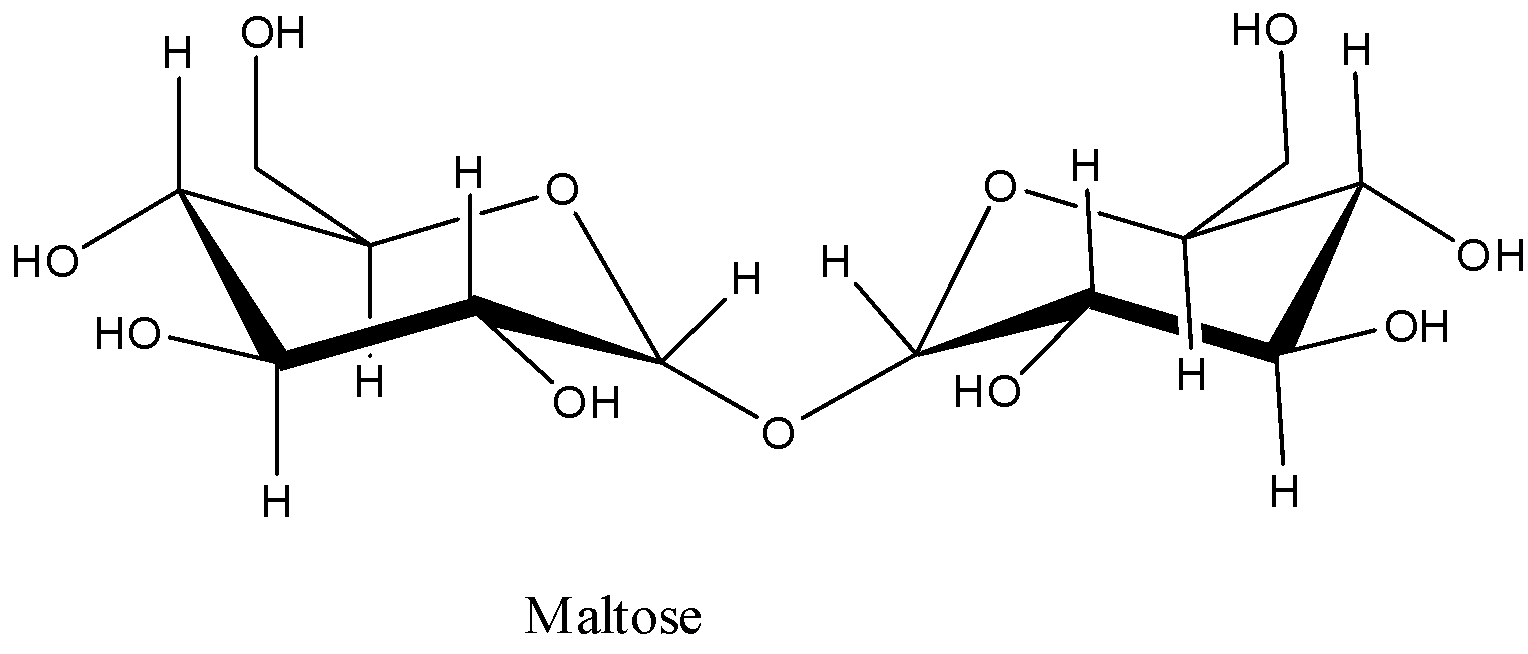
On hydrolysis of maltose, the \[\alpha \]-(1,4) glycosidic linkage undergoes cleavage and forms two units of \[\alpha \]-D-Glucose molecules as the products.
The hydrolysis of the maltose is as follows.
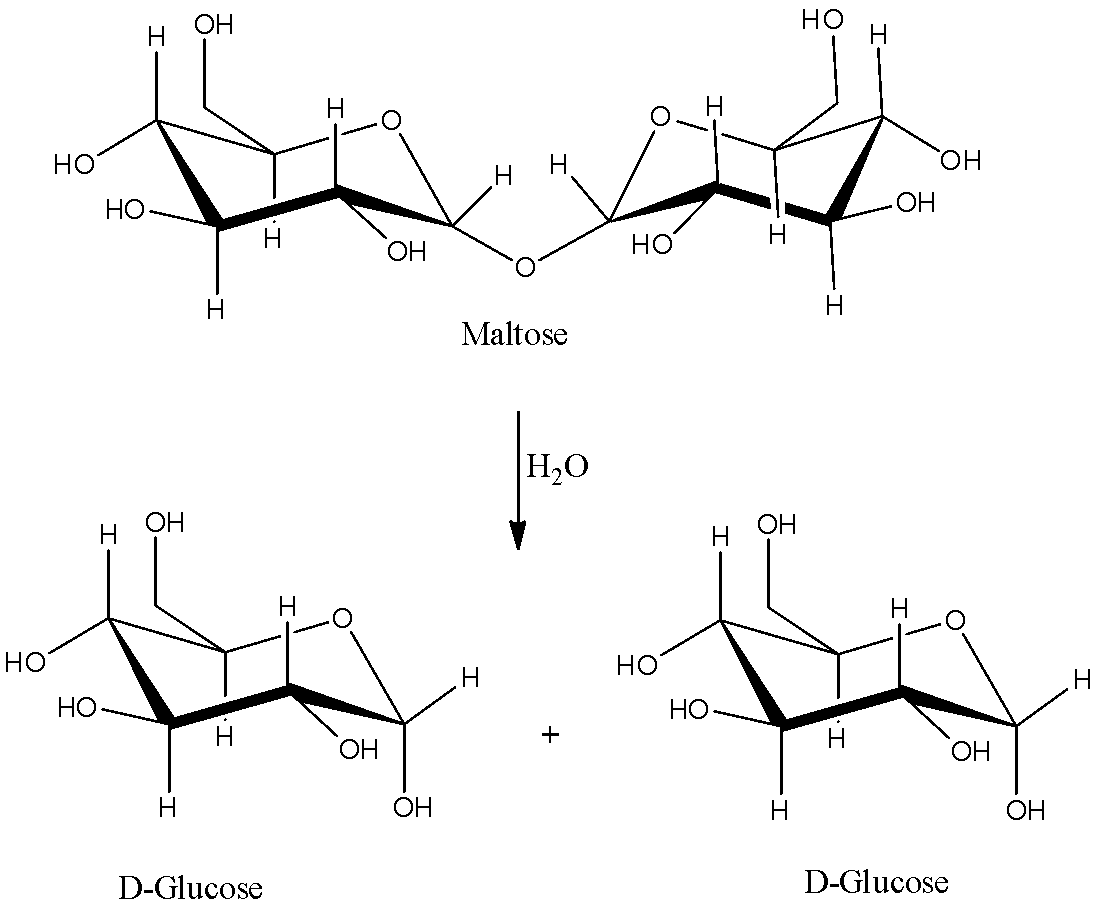
So, the products formed after hydrolysis of maltose are two D- glucose units.
Additional information:
Maltose is an intermediate compound formed in the intestinal digestion of glycogen and starch by an enzyme called amylase.
Maltose is found in germinating grains and in vegetables.
Maltose tastes like caramel, and maltose is used in bakeries, sweets, alcoholic drinks, and in infant food.
Maltose produces four calories of energy per one gram, which is equal to the same energy released by glucose or sucrose.
Maltose is less sweet than the table sugar and fructose. So, it is used in hard candy and in frozen desserts.
Note: If any molecule contains two carbohydrate units then it is called as disaccharide. Maltose is a disaccharide. Sucrose is also a disaccharide containing glucose and fructose.
Three carbohydrate units – Trisaccharide.
More than three units – Oligosaccharide.
Complete answer:
Maltose is a disaccharide consisting of two molecules of glucose with \[\alpha \]-(1,4) glycosidic linkage.
The structure of the maltose is as follows.

On hydrolysis of maltose, the \[\alpha \]-(1,4) glycosidic linkage undergoes cleavage and forms two units of \[\alpha \]-D-Glucose molecules as the products.
The hydrolysis of the maltose is as follows.

So, the products formed after hydrolysis of maltose are two D- glucose units.
Additional information:
Maltose is an intermediate compound formed in the intestinal digestion of glycogen and starch by an enzyme called amylase.
Maltose is found in germinating grains and in vegetables.
Maltose tastes like caramel, and maltose is used in bakeries, sweets, alcoholic drinks, and in infant food.
Maltose produces four calories of energy per one gram, which is equal to the same energy released by glucose or sucrose.
Maltose is less sweet than the table sugar and fructose. So, it is used in hard candy and in frozen desserts.
Note: If any molecule contains two carbohydrate units then it is called as disaccharide. Maltose is a disaccharide. Sucrose is also a disaccharide containing glucose and fructose.
Three carbohydrate units – Trisaccharide.
More than three units – Oligosaccharide.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




