
What are the products formed when phenol and nitrobenzene are treated separately with a mixture of concentrated sulfuric acid and concentrated nitric acid?
Answer
612k+ views
Hint: To answer this question you must know how benzene and nitrobenzene differ in reaction with a mixture of concentrated sulfuric acid and concentrated nitric acid. In these reactions, ring activation and deactivation plays an important role. Now you have to think about the formation of products.
Complete step by step answer:
When the mixture of concentrated sulphuric acid and concentrated nitric acid reacts together generates the nitronium ion, which acts as an electrophile in electrophilic aromatic substitution reaction.
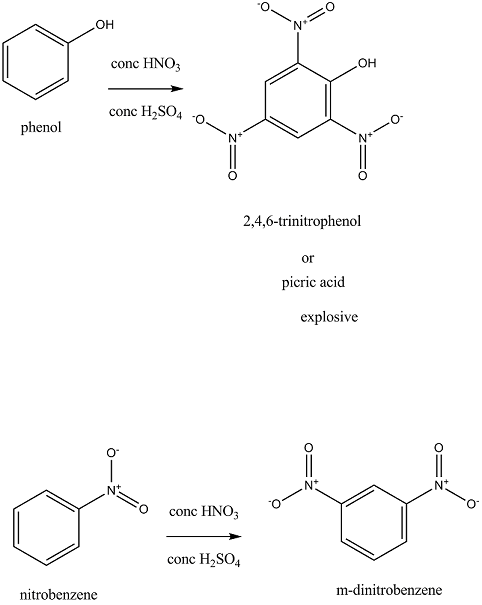
When phenol is treated separately with a mixture of concentrated sulphuric acid and concentrated nitric acid, 2,4,6-trinitrophenol ( also known as picric acid) is obtained. Hydroxyl group is an activating group here and so it is ortho, para directing. In this reaction conditions are so favorable that the nitro group is attached to all ortho and para positions.
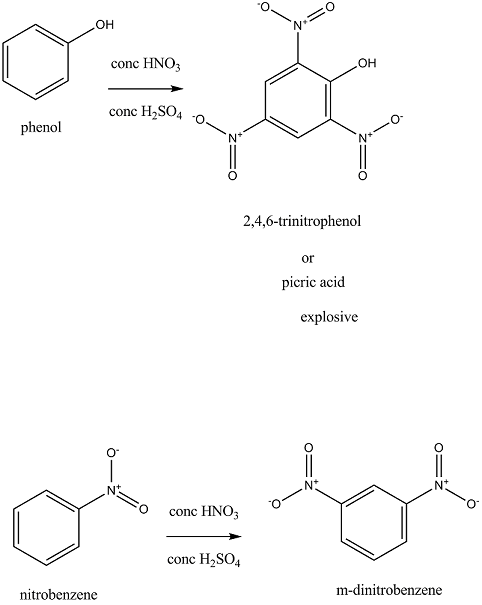
When nitrobenzene is treated separately with a mixture of concentrated sulphuric acid and concentrated nitric acid, m-dinitrobenzene acid is obtained. Nitro group is a deactivating group present here and so it is meta directing.
Therefore, we can conclude that picric acid and m-dinitrobenzene are the products formed when phenol and nitrobenzene are treated separately with a mixture of concentrated sulfuric acid and concentrated nitric acid.
Note: You should also know that the largest use of Picric acid has been in munitions and explosives. Explosive D, also known as Dunnite, is the ammonium salt of picric acid—more powerful but less stable than the more common explosive TNT.
Complete step by step answer:
When the mixture of concentrated sulphuric acid and concentrated nitric acid reacts together generates the nitronium ion, which acts as an electrophile in electrophilic aromatic substitution reaction.
When phenol is treated separately with a mixture of concentrated sulphuric acid and concentrated nitric acid, 2,4,6-trinitrophenol ( also known as picric acid) is obtained. Hydroxyl group is an activating group here and so it is ortho, para directing. In this reaction conditions are so favorable that the nitro group is attached to all ortho and para positions.

When nitrobenzene is treated separately with a mixture of concentrated sulphuric acid and concentrated nitric acid, m-dinitrobenzene acid is obtained. Nitro group is a deactivating group present here and so it is meta directing.

Therefore, we can conclude that picric acid and m-dinitrobenzene are the products formed when phenol and nitrobenzene are treated separately with a mixture of concentrated sulfuric acid and concentrated nitric acid.
Note: You should also know that the largest use of Picric acid has been in munitions and explosives. Explosive D, also known as Dunnite, is the ammonium salt of picric acid—more powerful but less stable than the more common explosive TNT.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




