
What are the major products formed in the following reaction?
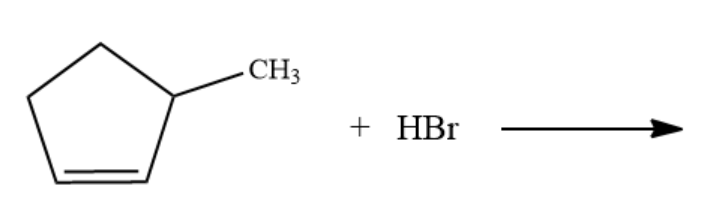
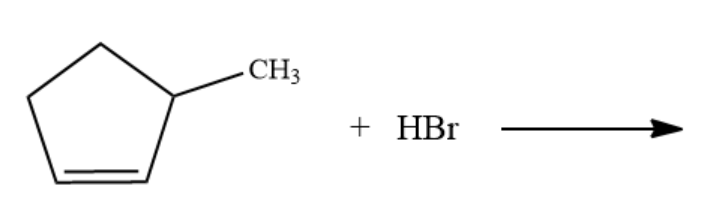
Answer
504k+ views
Hint: In the above reaction $ 1 - methylcyclopentene $ is reacting with HBr to give some product. The reaction taking place is an additional reaction where the halide part gets attached to the asymmetrical carbon. In this reaction, two products are obtained and the reaction will follow certain rules.
Complete answer:
In the above reaction, we see that $ 1 - methylcyclopentene $ is reacting with hydrobromic acid to give some product. $ 1 -$ methylcyclopentene is an alkene, and when any alkene is treated with HBr then it results in the formation of alkyl bromide.
The product formed in the above reaction is as follows:
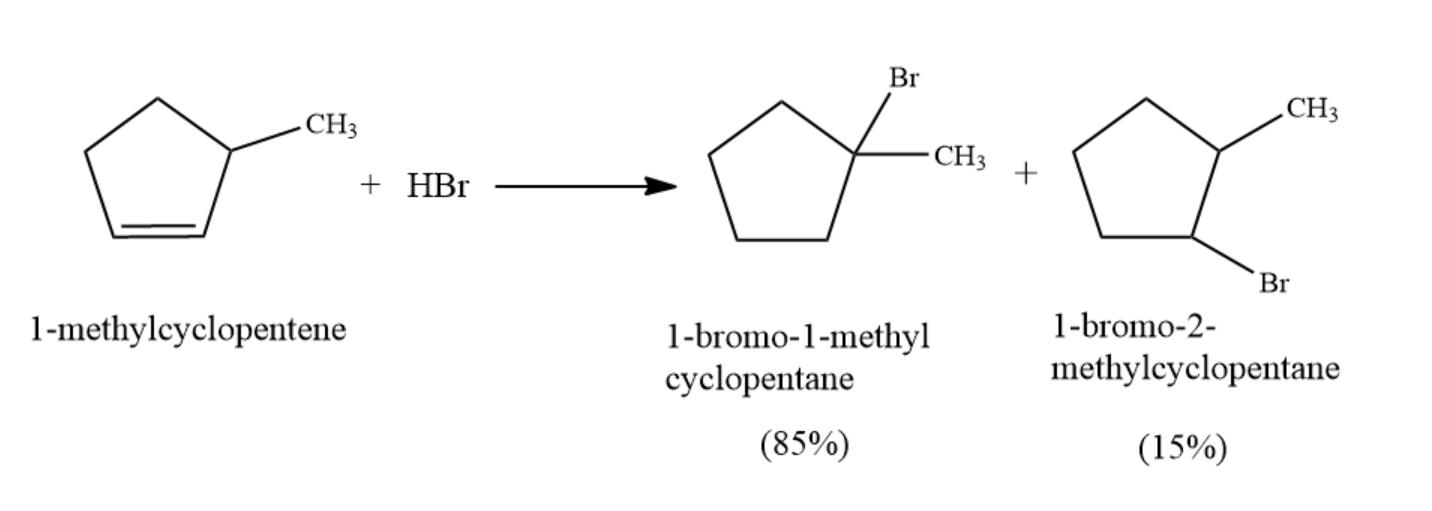
So it can be seen that the major product in the following reaction is $ 1 - Bromo - 1 - methyl cyclopentane. $
The reaction taking place here between $ 1 - methylcyclopentene $ and HBr is an additional reaction. The reaction will occur via Markonikoff’s rule. And so the bromine will always end up at the more substituted carbon of the alkene.
In addition to reaction, any halogen can be used except fluoride. In these reactions, asymmetrical carbons are treated with HX (where X is any halogen bromine, chlorine, or iodine) and this results in the formation of a product that is alkyl halide. The reaction occurs via Markonikoff’s rule. According to the rule when a protic (HX) is added to an asymmetric alkene, the acidic hydrogen attaches itself to the carbon having a greater number of hydrogen substituents whereas the halide group attaches itself to the carbon atom which has a greater number of alkyl substituents.
Note:
In such a reaction whenever there is the use of peroxide then the reaction occurs via anti-markovnikov's rule. This is exactly the opposite of that of Markonikoff’s rule. The reactions that occur via anti-markovnikov's rule are called free radical addition reactions.
Complete answer:
In the above reaction, we see that $ 1 - methylcyclopentene $ is reacting with hydrobromic acid to give some product. $ 1 -$ methylcyclopentene is an alkene, and when any alkene is treated with HBr then it results in the formation of alkyl bromide.
The product formed in the above reaction is as follows:

So it can be seen that the major product in the following reaction is $ 1 - Bromo - 1 - methyl cyclopentane. $
The reaction taking place here between $ 1 - methylcyclopentene $ and HBr is an additional reaction. The reaction will occur via Markonikoff’s rule. And so the bromine will always end up at the more substituted carbon of the alkene.
In addition to reaction, any halogen can be used except fluoride. In these reactions, asymmetrical carbons are treated with HX (where X is any halogen bromine, chlorine, or iodine) and this results in the formation of a product that is alkyl halide. The reaction occurs via Markonikoff’s rule. According to the rule when a protic (HX) is added to an asymmetric alkene, the acidic hydrogen attaches itself to the carbon having a greater number of hydrogen substituents whereas the halide group attaches itself to the carbon atom which has a greater number of alkyl substituents.
Note:
In such a reaction whenever there is the use of peroxide then the reaction occurs via anti-markovnikov's rule. This is exactly the opposite of that of Markonikoff’s rule. The reactions that occur via anti-markovnikov's rule are called free radical addition reactions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




