
What are the main energy levels where the valence electrons belong?
Answer
524.7k+ views
Hint:One must know an electron shell, also known as the principal energy stage, is the orbit of one or more electrons around the nucleus of an atom in chemistry and atomic physics. The "1 shell" (also known as the "K shell") is the nearest to the nucleus, followed by the "2 shell" (or "L shell"), and finally the "3 shell" (or "M shell"), and so on, as you get further away from the nucleus. Valence electrons are the electrons that make up an atom's outermost shell.
Complete answer:
When a quantum mechanical device or particle is bound—that is, spatially constrained—it can only take on discrete energy values known as energy levels. Energy levels (also known as electron shells) are described as fixed distances from an atom's nucleus where electrons can be found. Electrons are negatively charged particles that float around the positive nucleus in the middle of an atom. The steps of a staircase are similar to energy levels. You can stand on one of the steps, but not in the middle.
Electrons are the same way. They can only occupy one energy level at a time, not the space between them.The first four energy levels of an atom are depicted in the model below.Energy level I (also known as energy level K) electrons have the least amount of energy.Higher-level electrons have more energy when you get farther away from the nucleus, and their energy increases by a defined, distinct number.
If electrons consume this amount of energy, they will hop from one energy level to the next. As electrons move from a higher to a lower energy level, they emit energy, which is usually in the form of light. This explains the above-mentioned fireworks. Electrons gain energy and leap to higher energy levels as fireworks explode. They release the energy as light as they return to their original energy levels. Electrons are arranged differently in different atoms.
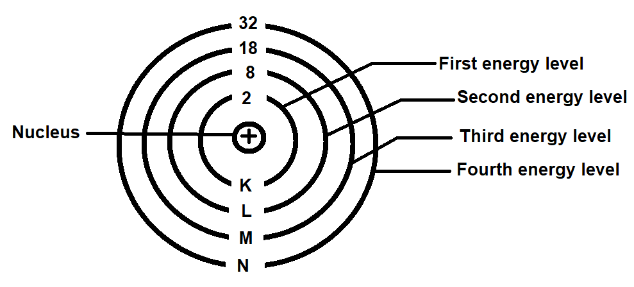
In the diagram above, the numbers 2, 8, 18, and 32 represent the total number of potential electrons that could be kept in each energy level.Hydrogen atoms are the smallest atoms.There is just one electron in them. The first energy level is occupied by the single electron. More electrons are found in larger atoms.
Electrons are often applied to the lowest energy level first, until it has the highest possible number of electrons. Then, before the next higher energy level is absolute, electrons are applied to it, and so on.In the diagram above, the maximum number of electrons possible for the first four energy levels is shown.
Energy level I, for example, can hold a maximum of two electrons, while energy level II can hold up to eight electrons. At a given energy level, the maximum number is determined by the number of orbitals.Electrons in an atom's outermost energy level have a unique meaning. These electrons are known as valence electrons, and they control many of an atom's properties.
An atom is most stable when it has as many electrons as it can bear in its outermost energy level.Electrons in an atom's outermost energy level have a unique meaning. These electrons are known as valence electrons, and they control many of an atom's properties. When an atom's outermost energy level has as many electrons as it can bear, it is the most stable.
Note:Energy levels (also known as electron shells) are described as fixed distances from an atom's nucleus where electrons can be found. Electrons at higher energy levels provide more energy when you move away from the nucleus. . Electrons are always applied to the lowest energy level first, until that level is complete, and then to the next higher energy level, until that level is full, and so on. The number of orbitals determines the maximum number of electrons at a given energy level.
Complete answer:
When a quantum mechanical device or particle is bound—that is, spatially constrained—it can only take on discrete energy values known as energy levels. Energy levels (also known as electron shells) are described as fixed distances from an atom's nucleus where electrons can be found. Electrons are negatively charged particles that float around the positive nucleus in the middle of an atom. The steps of a staircase are similar to energy levels. You can stand on one of the steps, but not in the middle.
Electrons are the same way. They can only occupy one energy level at a time, not the space between them.The first four energy levels of an atom are depicted in the model below.Energy level I (also known as energy level K) electrons have the least amount of energy.Higher-level electrons have more energy when you get farther away from the nucleus, and their energy increases by a defined, distinct number.
If electrons consume this amount of energy, they will hop from one energy level to the next. As electrons move from a higher to a lower energy level, they emit energy, which is usually in the form of light. This explains the above-mentioned fireworks. Electrons gain energy and leap to higher energy levels as fireworks explode. They release the energy as light as they return to their original energy levels. Electrons are arranged differently in different atoms.

In the diagram above, the numbers 2, 8, 18, and 32 represent the total number of potential electrons that could be kept in each energy level.Hydrogen atoms are the smallest atoms.There is just one electron in them. The first energy level is occupied by the single electron. More electrons are found in larger atoms.
Electrons are often applied to the lowest energy level first, until it has the highest possible number of electrons. Then, before the next higher energy level is absolute, electrons are applied to it, and so on.In the diagram above, the maximum number of electrons possible for the first four energy levels is shown.
Energy level I, for example, can hold a maximum of two electrons, while energy level II can hold up to eight electrons. At a given energy level, the maximum number is determined by the number of orbitals.Electrons in an atom's outermost energy level have a unique meaning. These electrons are known as valence electrons, and they control many of an atom's properties.
An atom is most stable when it has as many electrons as it can bear in its outermost energy level.Electrons in an atom's outermost energy level have a unique meaning. These electrons are known as valence electrons, and they control many of an atom's properties. When an atom's outermost energy level has as many electrons as it can bear, it is the most stable.
Note:Energy levels (also known as electron shells) are described as fixed distances from an atom's nucleus where electrons can be found. Electrons at higher energy levels provide more energy when you move away from the nucleus. . Electrons are always applied to the lowest energy level first, until that level is complete, and then to the next higher energy level, until that level is full, and so on. The number of orbitals determines the maximum number of electrons at a given energy level.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




