
What are the IR absorption bands and NMR data of triphenylmethanol?
Answer
531.9k+ views
Hint: We have to know that the IR assimilations happen at resounding frequencies that match the vibrational recurrence and are influenced by the state of the sub-atomic potential energy surfaces, nuclear masses and the related vibronic coupling. Infrared dynamic vibrations cause the groups found in an infrared range.
Other than recognizable proof, NMR spectroscopy gives itemized data about the design, elements, response state, and compound climate of atoms. The most widely recognized sorts of NMR are proton and carbon-13 NMR spectroscopy, yet it is relevant to any sort of test that contains cores having turns.
Complete step by step answer:
We can see the structure of triphenylmethanol.
We can draw the structure of triphenylmethanol as,
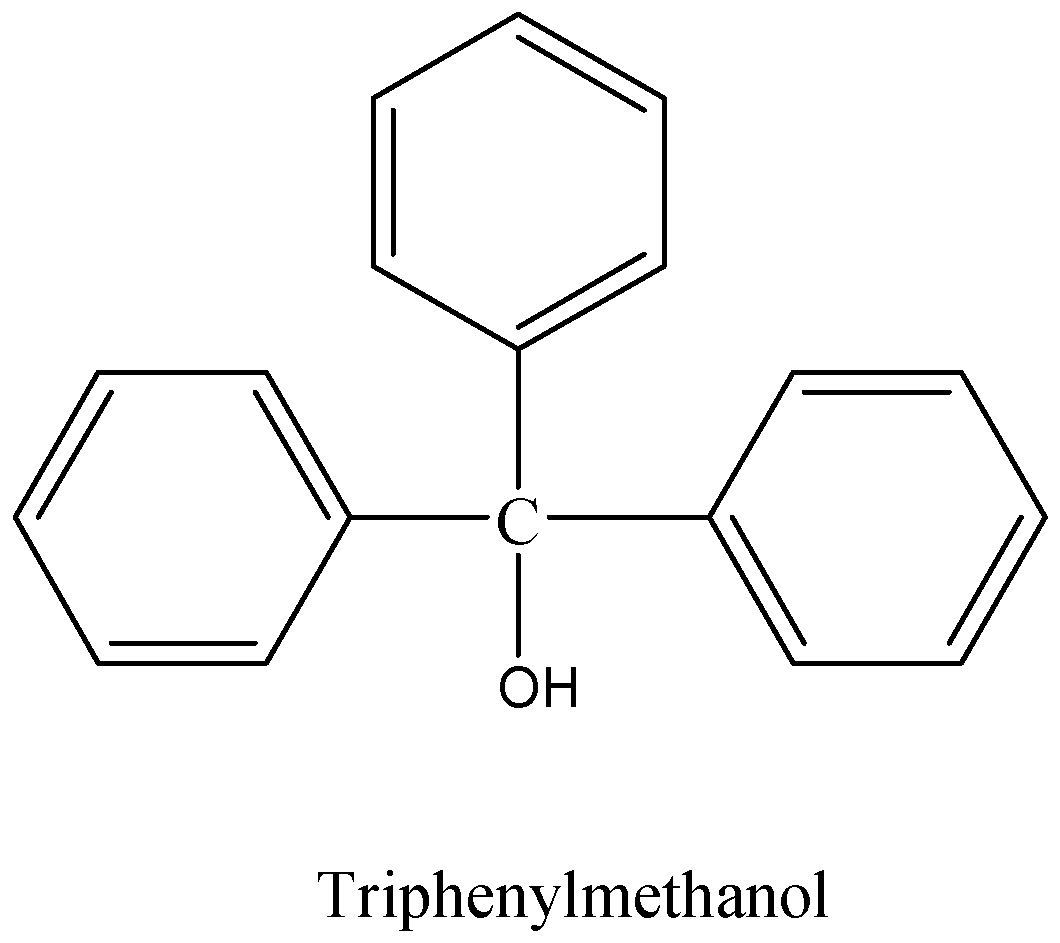
The chemical formula of the tri-phenyl methanol is, ${C_{19}}{H_{16}}O$.
Neither the NMR nor theIR information of triphenylmethanol would be at all conclusive or helpful. In the ${}^1H$ NMR range, you may see the top due to OH some place; mind you this is a low power top. In the IR range, there is nothing.
In the event that as a scientist you were approached to distinguish this material, you would make a couple of subordinates of the liquor, and contrast the dissolving focuses and the writing.
Note: We have to know that the IR spectroscopy regularly used to recognize structures in light of the fact that practical gatherings bring about trademark groups both, as far as force, and position. The places of these groups summed up in connection tables as demonstrated underneath.
NMR spectroscopy is the utilization of NMR wonders to consider the physical, compound, and organic properties of issue. Physicists use it to decide subatomic character and design. Clinical professionals utilize attractive reverberation imaging (MRI), a multidimensional NMR imaging method, for indicative purposes.
Other than recognizable proof, NMR spectroscopy gives itemized data about the design, elements, response state, and compound climate of atoms. The most widely recognized sorts of NMR are proton and carbon-13 NMR spectroscopy, yet it is relevant to any sort of test that contains cores having turns.
Complete step by step answer:
We can see the structure of triphenylmethanol.
We can draw the structure of triphenylmethanol as,

The chemical formula of the tri-phenyl methanol is, ${C_{19}}{H_{16}}O$.
Neither the NMR nor theIR information of triphenylmethanol would be at all conclusive or helpful. In the ${}^1H$ NMR range, you may see the top due to OH some place; mind you this is a low power top. In the IR range, there is nothing.
In the event that as a scientist you were approached to distinguish this material, you would make a couple of subordinates of the liquor, and contrast the dissolving focuses and the writing.
Note: We have to know that the IR spectroscopy regularly used to recognize structures in light of the fact that practical gatherings bring about trademark groups both, as far as force, and position. The places of these groups summed up in connection tables as demonstrated underneath.
NMR spectroscopy is the utilization of NMR wonders to consider the physical, compound, and organic properties of issue. Physicists use it to decide subatomic character and design. Clinical professionals utilize attractive reverberation imaging (MRI), a multidimensional NMR imaging method, for indicative purposes.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




